
https://en.wikipedia.org/wiki/Cool_(aesthetic)
https://www.youtube.com/watch?v=Af3ZG47oT7I&ab_channel=Vsauce
On a coolness scale, how cool are you today? If you don't know, that's okay because how cool you feel is subjective and there isn't any way to objectively measure "coolness". In your opinion, what things constitutes as 'cool'? If your responses include sunglasses, laidback attitude, relaxed pose, trendy fashion, hiphop music, dance/techno, anything hipster, you are amongst the majority. But why? What do these things have in common that makes them 'cool'? We first need to ask ourselves this question.
What is cool?
- Because the connotations continue to evolve, as well as the subjective nature of the term, there is no single definition of 'cool'.
- It is considered to be an aesthetic of attitude, behaviour, appearance and style that is generally admired. It tends to associate with composed and self-control, as well as a way to express one's admiration or approval.
- A self-conscious poise in behaviour characterised by a set of discernible bodily movements, postures, facial expressions and voice modulations that demonstrates social value within the peer context.
- It was once an attitude promoted by rebels and underdogs, such as prisoners, slaves, bikers and political protestors etc. This is due to open rebellion being a punishable act, hence they concealed their defiance behind a wall of ironic detachment, and distanced themselves from authority rather than directly faced them.
- Warren & Campbell (2004) defined coolness as a positive trait based on the inference that a cultural object is autonomous appropriately i.e. the person or bran isn't constrained by the norms, expectation of beliefs of others.
- Robert Thompson (1973) pointed out that "cool" describes a general state of well-being, a transcendent, internal state of peace and serenity. Furthermore, it may refer to a lack of conflict, a state of harmony and balance. e.g. "The land is cool.", "A cool [spiritual] heart."
- People who "keep their cool" are composed and don't show much excitement.
- People who are "cool with that" express their agreement or assent for a particular idea of interest.
- Nick Southgate stated that the term 'cool' was sought by product marketing firms and idealised by teenagers as a shield against racial oppression or political persecution and source of constant cultural innovation.
 |
| Millennial (Generation Y) cool fashion |
- The concept of "cool fashion" evolved from the 1940s that began with USA's mass-produced "ready-to-wear" fashion, which resulted in conventional outfits that depicted one's fixed social role in society.
- During the baby boomer generation (1940s and 50s), subcultures such as the hipsters (hippies) felt repressed by the prevalent conservative ideology towards conformity and rebellion.
- Dick Pountain described hipster's fashionable dress sense as "cool" due to its noticeable divergence from the standard uniformity of dress and mass-production of dress, produced by the totalitarian system of fashion.
- Lauren Whitley (2013) described various different styles that highlighted bold colors such as the "Trippy Hippie", the "Fantasy Hippie", the "Retro Hippie", the "Ethnic Hippie", and the "Craft Hippie".
- According to the strain theory, the hippie fashion is mostly handmade that passively rebelled against consumerism, which is considered "cool". Due to their disengagement, there was limited self-critique because their facade filtered negative thoughts of worthlessness, which fostered the opportunity for self-worth.
- Dick Pountain (2000) stated the hippie "rebels" of the late 1960s filled the senior executive roles of the business sector and of the fashion industry, which incorporated the concept of "dressing cool" into the mainstream culture to become a prominent ideology in the 21st century. Nevertheless, the definition of "cool" in the fashion sense changed to one that attempted to conceal their insecurities with confidence.
- During the 1990s and 21st century, the "fashion-grunge" style masked people's financial insecurities in order to conform in a certain group based on a unique piece of clothing. For example, a person who presents Marc Jacobs' combined "fashion-grunge" style wears "a little preppie, a little grunge and a little couture". This emanated mystery and awkwardness regarding their internal feelings.
 |
| Hippies in the 1960s |
- The term "cool" is generally a positive epithet or interjection, which comprises of a range of related adjectival meanings.
- e.g. A cool person is viewed as being themselves and not concerned about other's perceptions of their opinions and behaviours.
 |
| This is a timeline of "cool", adapted from Dick Pountain and David Robins, Cool Rules: Anatomy of an Attitude. |
- Aware of and responsive to the latest developments particularly in fashion and entertainment i.e. au courant, downtown, groovy, hip, trendy
- Of the very best kind i.e. awesome, boss, dope, down, cracking, dynamite, excellent, fabulous, hype, marvellous, out-of-sight, radical, prime, stellar, sensational, unsurpassed
Where did "cool" originate?
i. Africa & the African diaspora- In 1973, Art History professor Robert Farris Thompson, from Yale University, conjectured that Itutu (translated to "mystic coolness") was 1 of 3 pillars of a religious philosophy emerged in the 15th century by Yoruba and Igbo civilizations of West Africa.
- It was discovered Itutu (Cool) associated with conciliation and gentleness of character, of generosity and grace, and the ability to quell arguments and conflicts, as well as physical beauty. In Yoruba culture, Itutu associated with water, because the concept of coolness maintained its physical nuance of temperature.
- The Gola people of Liberia defined "cool" as the ability to remain mentally calm or detached, in a spiritually fashion, from one's one's circumstances, to be nonchalant in emotionalist or eager situations. Murphy & Sanford suggested "cool" was related to the deity Òsun of the Yoruba religion.
- Robert Thompson (1979) found a number of similarities and differences between Africans' and Europeans' notions of coolness. They were both similar in terms of self-control and imperturbability, but differed in terms of the African cultural and diaspora influence. Moreover, he stated that cool was instrumental in traditional African cultures spiritually, which wasn't observed in early Western contexts.
- In 2007, Ronald Perry listed several words and expressions in the Standard English slang that were derived from African-American Vernacular English including the contemporary meaning of the word "cool". Popularised in jazz circles by tenor saxophonist Lester Young in the 1940s, it gave birth to the "Bohemian", or beatnik, culture.
- Subsequently, the style of cool jazz emerged on stage, which accentuated a restrained, laid-back solo style.
- Expressions such as "Don't blow your cool", or later, "chill out", and the use of "chill" portrayed inner contentment or restful repose.
- In her book What is Cool?: Understanding Black Manhood in America, Mariene Kim Connor defined cool as "the silent and knowing rejection of racist oppression, a self-dignified expression of masculinity developed by black men denied mainstream expressions of manhood".
- She pointed out that mainstream perception of cool is narrow and distorted, often perceived as style or arrogance, instead of an action to earn respect.
- Richard Major (1992) thought black American men demonstrated coolness in order to display a powerful appearance that help retained a social audience, which may have origins in slavery.
French fashion designer Christian Lacroix: "The history of cool in America is the history of African-American culture."
 |
| Malcolm X's cool pose. |
- In urban black male culture, cool may have helped black men reduce stressed triggered by social oppression, rejection, and racism.
- Majors and Billson asserted that "cool" provided black men a sense of control, strength, confidence and stability, which allowed them to handle the closed doors and negative messages of the "generalised other". In addition, they associated black manhood with the perils of discrimination, negative self-image, guilt, shame and fear.
- A 2004 study on African-American culture-related movements discovered a correlation between the "cool pose" and decreased academic achievement, elevated aggression, and higher demand for special education services than students with standard movement styles, irrespective of race or other academic indicators.
- This problem of stereotyping and discrimination associated with the cool pose sparks questions of assimilation and accommodation of different cultural values.
- In Japan, "cool" is synonymous with aesthetical concepts such as iki and sui, which are traditional commoners' aesthetic ideals that developed in Edo.
- This is a debate regarding the ethic of the Samurai caste in Japan, warrior castes in India and East Asia resembling coolness. e.g. Akira Kurosawa's films such as The Seven Samurai, Yojimbo, and The Hidden Fortress. Fun fact: The Hidden Fortress inspired George Lucas's Star Wars, where aspects from the samurai inspired the creation of the Jedi Knights.
- In Hannah Beech's Time Asia article, "The Birth of Cool", she depicts Asian cool as "a revolution in taste led by style gurus who are redefining Chinese craftsmanship in everything from architecture and film to clothing and cuisine". Furthermore, she referred it as a modern aesthetic inspired by both Ming-era minimalism and the ability to be meticulous.
- Professor of Human Geography at the University of Leeds, Paul Waley, regarded Tokyo as one of the world's capitals of cool, which is comparable to New York, London and Paris.
- The Washington Post labelled Tokyo "Japan's Empire of Cool" and Japan "the coolest nation on Earth". It referred to Japan's "gross national cool" that may be a factor behind its economic growth and societal buoyancy.
- In his 2002 Foreign Policy magazine article, journalist Douglas McGray coined the term "gross national cool" to encapsulate Japan's emergence as a cultural juggernaut with references to its architecture, fashion, pop music, consumer electronics, traditional food and art.
- Known as sprezzatura, "aristocratic cool" existed in Europe for centuries in the form of amorality and love or illicit pleasures behind closed doors. Example paintings that illustrate sprezzatura include Raphael's Portrait of Baldassare Castiglione and Leonardo da Vinci's Mona Lisa.
- That Italian word was coined by Baldassare Castiglione in his 1528 book The Book of the Courtier. He defined it as "a certain nonchalance, so as to conceal all art and make whatever one does or says appear to be without effort and almost without any thought about it". In other words, it means disdain and detachment, or the art of abstaining from the appearance of presenting oneself in a certain way.
- In William Shakespeare's Midsummer Night's Dream, "cool reason" was used to distinguish the tangible fantasises of lovers and madmen. In addition, he wrote "O gentle son, upon the heat and flame of thy distemper, sprinkle cool patience" in Hamlet, as well as described the antagonist Iago in Othello musing about "reason to cool our raging motions, our carnal stings, our unbitted lusts."
- In the aftermath of WWI, modern European cool was advanced by avant-garde artists, mostly Dadaists such as Arthur Cravan and Marcel Duchamp. Pountain & Robins (2000) suggested this was a self-conscious revolutionary that aimed to scandalise bourgeoisie by mocking their culture, sexuality and political moderation.
- One archetype of this inter-war cool is well-known committed Communist and a philandering cynic Berthold Brecht. He demonstrated his "cool" attitude through his most famous character named Macheath or "Mackie Messer" (Mack the knife) in The Threepenny Opera. Mackie is a nonchalant, smooth-talking gangster, expert with the switchblade who personifies the bitter-sweet strain of cool.
- Peter Stearns (1994) believed cool was a privilege for bohemian milieus such as Brecht's. Cool irony and hedonism was demonstrated by cabaret artistes, ostentatious gangsters and rich socialites, which were depicted in Evelyn Waugh's Brideshead Revisited and Christopher Isherwood's Goodbye to Berlin. This may sow the seeds of a cool outlook among that inter-war generation.
- After WWII, there was an intimate interaction between British, German and French cultures with American culture. Europeans associated the American GIs' relaxed, easy-going manner with liberation, hence Lucky Strikes, nylons, swing and jazz, i.e. the American Cool.
- Cool or hip behaviours associated with hangouts, pursuit of sexual liaisons, selection of the appropriate attitude of narcissistic self-absorption, and the desire to escape the mental straitjacket of all ideological causes.
- Since the late 1940s, the embrace of popular culture by young people worldwide appalled the paternalistic elites who still ruled the official culture such as the French intelligentsia. Nevertheless, the British educated classes felt pompously indifferent that was reminiscent of an older aristocratic cool.
- The earnestness of socialist propaganda and socialist realism in art survived and existed behind the Iron Curtain throughout the 20th century.
- In the Polish industrial city Łódź, jazz was labelled as "the forbidden music", which served Polish youth in the 1950s. It provided both personal diversion and subterranean resistance to the culture Polish youth viewed as oppressive.
- James Sloan (1996) explained a number of clubs featured live jazz performances in a smoky, sexually charged atmosphere that provided a suitable setting for puritanical values and monumental art of Marxist officialdom.
- Examples of Polish cool artists such as Andrzej Wajda, Roman Polanski, and other graduates of the National Film School in Łódź, as well as the novelist Jerzy Kosinski.
- In Prague, cool flourished in the faded Art Deco splendour of the Café Slavia.
- After the defeat of the Prague Spring by the Soviets in 1968, part of the dissident underground labelled itself the "Jazz Section".
What are the theories of "cool"?
- This theory defines coolness as a dynamic, subjective, socially-constructed trait, therefore coolness may be in the eye of the beholder. It hypothesises each person's perception of things such as other people, products, brands or activities in terms of coolness may be dependent on their "autonomy".
- It conjectures the level of autonomy that leads to coolness up to a certain extent. For instance, inappropriate levels of autonomy may not result in perceptions of coolness.
- Warren & Campbell (2014) proposed a direct relationship between the perceptions of societal institutions and authority of being unjust or repressive and the perceptions of coolness at higher levels of autonomy than those less critical of social norms and authority.
- Heath & Potter (2004) argued coolness is a relative concept that exists only when compared to things considered less cool. According to the book The Rebel Sell, the concept of cool emerged from the need for status and distinction. This may have triggered situations similar to an arms race, in which coolness is sustained by collection action.
- This theory suggests cool is a real but unknowable property, which exists that requires one to seek after.
- According to the 2013 New Yorker article "The Coolhunt", cool contains 3 characteristics:
- This theory suggested cool can be exploited by a top-down process called the Merchants of Cool, who sell popular culture and capitalise off of trends and subcultures.
- Examples of the "Merchants of Cool" include record company executives, sneakers and fashion company branders and merchandisers, who are usually young adults.
- A 2016 Medium report described cool as becoming "the central ideology of consumer capitalism", which motivated young people and adults to conform to the mainstream and attach to trends and to purchase products and/or brands that give off a cool appearance.
- In the 1960s, cool was sold when menthol cigarettes were marketed to African Americans. In 2004, over 70% of African American smokers preferred menthol cigarettes, compared with 30% of Caucasian American smokers.
- This demonstrated the tobacco industry manipulating the burgeoning black, urban, segregated, consumer market in cities at that time. A Fast Company magazine claimed a number of large companies began "outsourcing cool" by paying other "smaller, more-limber, closer-to-the-ground outsider" companies in order to keep a tab on customers' rapidly changing tastes and demands.
- Grant McCracken (2009): "If status is about standing, cool is about standing free."
- Lewis MacAdams (2007): "Cool is a knowledge, a way of life."
- Marcel Dansel: "Cool is an age-specific phenomenon, defined as the central behavioural trait of teenagerhood."
- Robert Farris Thompson (1983): "Coolness is the proper way you represent yourself to a human being."
- In William Gibson's novel Spook Country (2007), Bigend equates cool with a sense of exclusivity.
- In Terry Pratchett's novel Lords and Ladies (1992), the Monks of Cool asked the novice to select the coolest garment from a room full of clothes in their passing-out test. The correct answer is "Hey, whatever I select", which indicates that cool is primarily an attitude of self-assurance.
- Warren & Campbell (2014): "Coolness is a subjective and dynamic, socially constructed positive trait attributed to cultural objects (people, brands, products, trends, etc.) inferred to appropriately autonomous."

During the dead of winter, we all desire to be as snug as a bug in a rug. If you plan to go for a walk outside your warm home and into the snow-covered winter wonderland without wearing proper clothing and footwear, you would be putting yourself on thin ice and a snowball's chance in hell of returning home alive and unaffected. For those living in the northern hemisphere, you would associate snow with Christmas because it is winter between December and February in USA and Canada. This association is reinforced by the plethora of popular Christmas movies and cartoons that feature snowfall such as Merry Christmas Charlie Brown, Home Alone 1/2/3, Santa Clause, The Polar Express, Elf, The Grinch, Scrooge, Mickey's Christmas Carol, White Christmas, Die Hard and Bad Santa. So that makes North America the COOL-est continent in the world.
- Cold is defined as the presence of low atmospheric temperature, however the sensation of cold is subjective.
- Shivering
- Disruption to blood circulation e.g. Vasoconstriction
- Increased metabolism
- Freezing of extracellular water
- Destruction of tissue
- Skin discolouration, swelling, blisters, and haemorrhage
- Frostbite
- Chilblains or death to certain body parts
- Sepsis
- Hypothermia
- Also called shuddering, shivering is an important bodily function of warm-blooded animals that activates when exposed to cold environments.
- When your body's core temperature decreases, the shivering reflex is triggered to restore homeostasis. This involves your somatic skeletal muscles involuntarily trembling in order to generate warmth by expending energy.
- Nakamura & Morrison (2011) found the dorsomedial hypothalamus (DMH) or rostral raphe pallidus nucleus (rRPa) were involved in not only the shivering reflex, but also the brown adipose tissue (BAT) temperature thermogenic, tachycardic and pressor responses.
- The DMH is usually inhibited by a heat centre called the anterior hypothalamic-preoptic area, but excited by signals from the spinal cord and skin receptors during a cool environment.
(Left) In a warm environment
1. Cutaneous warm receptors are activated upon exposure to heat.
- When your skin is exposed to extreme cold, it freezes it and other tissues, resulting in frostbite. Symptoms are observable in the fingers, toes, nose, ears, cheeks and chin areas.
- Frostbite has been known as early as 400 BCE when the Greeks discovered the phenomenon. Researchers dated frostbite in humans back approximately 5,000 years in an Andean mummy.
- Handford et al. (2014) stated the first instance of mass frostbite was documented by Napoleon's Army in the early 1800s.
- Zafren (2013) estimated around 1 million combatants of World War I, World War II, and the Korean War suffered from frostbite.
- Superficial, surface skin damage that is temporary
- Loss of feeling in the skin
- Affected skin feels numb, swollen, with a reddened border
- After a few weeks of injury, the skin's surface may peel off.
- Clear blisters early on in the skin
- Hardening of the skin surface
- After several weeks of injury, the hardened and blistered skin dries, blackens and sloughs.
- Lasting cold sensitivity and numbness develops.
- Freezing of tissue layers beneath the skin surface
- Blood blisters and "blue-grey discolouration of the skin surface".
- After weeks of injury, persistent pain and a eschar (blackened crust) develops.
- Long-term ulceration
- Damage to growth plates
- Affected areas below the skin include the muscles, bones and tendons
- Colourless appearance on the skin
- Hard texture
- Painless rewarming
- Skin blackens and mummified
- Autoamputation
- Exposure to cold through geography, occupation and/or recreation.
- Inadequate clothing and shelter
- Impairment to the body's ability to retain heat and warmth
- Immobility
- Physical stress (such as malnutrition or dehydration)
- Disorders and substances that impair circulation such as diabetes, Raynaud's phenomenon, tobacco, and alcohol use
- Homelessness and certain mental illnesses
- When the body cools, it triggers vasoconstriction. If temperatures decrease below -4 °C (25 °F), it produces ice crystals in the tissues, which results in cellular damage.
- They can damage blood vessels leading to the formation of scar tissue when fibroblasts replace the dead cells.
- This damages tissue through reperfusion injury that involves oedema, stasis and vasoconstriction.
- Platelet aggregation, blisters and vasospasms can also manifest.
- Prefreeze = Tissues cool without ice crystal formation
- Freeze-thaw = Ice crystals form, leading to cellular damage and cell death.
- Vascular stasis = Blood coagulation, or leakage of blood out of the vessels
- Late ischaemic = Inflammatory events, ischaemia, and tissue death
- Similar to frostnip, but lacks the ice crystal formation in the skin
- Whitening of the skin and numbness reverse quickly after rewarming.
- Trench foot = Damage to nerves and blood vessels leading to exposure to wet, cold (non-freezing) conditions. Early treatment can reverse these symptoms.
- Pernio or chilblains = Inflammation of the skin from exposure to wet, cold (non-freezing) conditions. They can appear as various types of ulcers and blisters.
- Bullous pemphigoid = A condition that causes itchy blisters over the body that can mimic frostbite. It does not require exposure to cold to develop.
- Levamisole toxicity = A vasculitis that can appear similar to frostbite, which is caused by contamination of cocaine by levamisole.
- Hypothermia
- Aspirin and ibuprofen can prevent clotting and inflammation.
- Assess for hypothermia and other life-threatening complications of cold exposure.
- Prior to treatment, increase the core temperature to above 35 °C.
- Consider administrating oral or intravenous (IV) fluid.
- Handford et al. (2014) recommended a partially or fully frozen portion of skin to be rewarmed in the hospital with a warm bath filled with povidone iodine or chlorhexidine antiseptic. This helps minimise damage to the affected tissue.
- Rewarming the affected skin to 37-39 °C would be ideal, which should take between 15 mins and 1 hour.
- Pain management is required since the rewarming process can be painful.
- TPA with heparin can be administered to those with potential for amputations and present themselves within 24 hours of injury.
- Bone scans or CT angiography can be used to assess scale of damage.
- Blood vessel dilating medications such as iloprost to prevent blood vessel blockage. This treatment is suitable for grades 2-4 frostbite, if administrated within 48 hours.
- Sympatholytic drugs are another option to treat peripheral vasocoonstriction that occur during frostbite.
- Lorentzen, Davis & Penninga (2020) found iloprost alone or combined with recombinant tissue plasminogen activator (rtPA) decreases amputation rate in cases of severe frostbite compared to buflomedil.
- Debridement or amputation of necrotic tissue is put on hold unless there is gangrene or systemic infection (sepsis).
- Handford et al. (2014) recommended fasciotomy for symptoms of compartment syndrome in order to preserve blood flow.
- Also known as pernio and chill burns, this medical condition features damage to capillary beds in the skin, particularly in the toes or fingers, which leads to blood perfusion into the adjacent tissue causing redness, itchiness, inflammation, and blisters.
- Predisposed individuals, especially women, exposed to cold and humidity are likely to experience chilblains. Chilblains can be idiopathic, with similar symptoms manifesting from other serious medical conditions.
- Chilblains can appear as a symptom in a number of conditions such as Raynaud syndrome, Aicardi–Goutières syndrome, erythromelalgia, frostbite, and trench foot, as well as connective tissue diseases such as lupus or vasculitis.
- During the 2020 pandemic, a number of older children and adolescents were reported to suffer from chilblain-like symptoms after being infected with COVID-19. However, there is uncertainty whether these symptoms are a delayed consequence of the viral infection itself, or at least partially, due to environmental factors during the pandemic.
- Blisters
- Burning and itching sensations
- Dermatitis
- Ulceration
- Erythema
- Pain
- Skin discolouration (red to dark blue)
- Soaking in warm water with Epsom salts for 15–20 minutes, 3–4 times a day.
- Topical steroid cream
- Vasodilators such as Nifedipine and Diltiazem.
- A mixture of friar's balsam and a weak iodine solution
- Avoiding the restriction of the affected area
- When your core body temperature drops below 35.0 °C (95.0 °F), you would experience hypothermia. The Swiss staging system categorises hypothermia according to their presenting symptoms.
- Between 1995 and 2004 in the USA, an average of 1560 cold-related emergency department visits occurred per year.
- Between 1999 and 2004, an average of 647 people died per year due to hypothermia.
- A 2018 study found 49% of the reported deaths due to hypothermia between 1999 and 2004 in the USA were aged 65+ years and roughly 66% were male.
- Furthermore, 63% of hypothermia deaths were not work-related and 23% of deaths occurred at home.
- Unsurprisingly, a majority of hypothermia cases are reported during the autumn and winter months.
- An estimated 300 British people die from hypothermia per year, whereas an estimated 8,000 Canadians die from hypothermia-related incidents every year.
- Hypothermia played a significant role in determining the outcome of historical military campaigns, expeditions, and sinking ships that resulted in high death toll.
- Hannibal's campaign during the Second Punic War (218 B.C.), Napoleon's Russian campaign in 1812, and the Russian regions throughout WWI and WWII, especially in the Battle of Stalingrad.
- The sinkings of the RMS Titanic, RMS Lusitania and MS Estonia.
- Antarctic explorers such as Ernest Shackleton's team, and Captain Robert Falcon Scott's team.
- Nazi human experimentation during WWII equivalent to medical torture.
- Since extreme hypothermia can suppress heart and brain function, doctors are recommended to avoid declaring a person dead early until their body can be rewarmed to a near optimal body temperature of at least 32°C (90 °F). Exceptions include obvious fatal injuries or a frozen incompressible chest.
- Brown et al. (2012) noted other exceptions including burial in an avalanche for more than 35 minutes and the mouth crammed with snow without a pulse, as well as blood potassium levels larger than 12 mmol/l.
- Aggressive treatment is required if patients have stiff pupils and are immovable.
- Bolte et al. (1988) stated that unconscious children who experienced near-drowning accidents in water near 0 °C (32 °F) require urgent CPR within an hour.
- This is due to decreased metabolism in colder waters, and energy conservatism in the brain in order to endure a longer period of hypoxia.
- Depending on a range of factors such as state of health, underlying conditions, availability of help and effectiveness and rapidness of treatment, it is estimated mortality from hypothermia ranges between 38% and 75%.
- During moderate and severe hypothermia, victims became disoriented, confused, and combative. They would unimaginably remove their clothing, which accelerates body heat loss. For example, Russian explorers on the Dyatlov Pass were hypothesised to have suffered from paradoxical undressing being perishing in the cold.
- It is estimated between 20 and 50% of hypothermia deaths attributed to paradoxical undressing.
- The exact cause of this strange phenomenon is currently unknown, though scientists suggested a number of theories such as the hypothalamus malfunctioning in the shivering conditions, loss of vasomotor tone (i.e. exhaustion and relaxation of the muscles responsible for contracting peripheral blood vessels) that lead to significant blood flow to the extremities, causing overheating.
-- Terminal burrowing
- Also known as "hide-and-die syndrome", terminal burrowing is defined as an apparent self-protective behaviour. Those affected would enter small, enclosed spaces, such as under the bed or behind wardrobes.
- In 2013, German researchers suggested an autonomous process of the brain stem is activated in the final state of hypothermia and generates a primitive and burrowing-like behaviour of protection, commonly observed in hibernating animals.
What are the causes of hypothermia?
 |
| There is a strong relationship between the rate of hypothermia and age in the USA. |
- Risk factors that decrease heat production, increase heat loss, or impair thermoregulation such as substance use disorders, homelessness, conditions that impair judgment (e.g. hypoglycaemia), extreme age, poor-quality clothing, chronic medical conditions (e.g. hypothyroidism and sepsis), and living in extreme cold environments.
- Hypothermia also coincides with major trauma, anorexia nervosa, and severe cases of sepsis.
- A 2018 study by BMJ found hypothermia cases in urban areas associated with chronic cold exposure, attributed to homelessness or immersion incidents involving drugs, alcohol or mental illness.
- A 2018 report found hypothermia cases in rural areas associated with comorbid health conditions, decreased independent movement, wilderness exploration, and outdoor water sport events.
- Alcohol consumption stimulates vasodilation and certain brain regions responsible for thermoregulation, which increases the risk of hypothermia.
- This also increases the perception of warmth, quietens the shivering response, and expends energy slated for assisting the body in generating heat.
- In 2012-13, 28,354 cases of hypothermia related to poverty in the UK were reported, which was a 125% increase from 2011-12.
- Some cases of hypothermia associated with lack of insulating shelter, expensive energy and power bills, as well as disability and pension-holders.
- Hypothermia is a limiting condition to swimming or diving in cold water since decreases in finger due to pain or numbness compromises general safety and work capacity, which consequently increases the risk of other injuries.
- Other factors that predispose to immersion hypothermia include dehydration, lack of rewarming efforts between repetitive dives, diving in cold, wet dry suit undergarments, sweating with work, and deficient thermal insulation, and inadequate physical conditioning.
- A 2007 study found immersing in water of temperatures of 10 °C (50 °F) can lead to mortality within a month, and within 15 minutes in water of temperatures of near freezing point.
- The cause of death via water immersion is typically the bodily reactions to heat loss and to freezing water, rather than hypothermia (loss of core temperature) itself. Vittone (2010) found the outcome of plunging into freezing water is generally cold shock within 2 minutes. This included uncontrolled rapid breathing, gasping, resultant water inhalation, significant vasodilation, and cardiac strain, which resulted in cardiac arrest and panic.
- Furthermore, mortality can occur due to cold incapacitation within 15-30 minutes of immersion in freezing water. This refers to inability of limb and hand control to swim, since the body shuts down the peripheral muscles of the limbs to protect its core.
- Heat loss occurs through the skin (90%) and lungs (10%) via convection, conduction, and radiation. Factors that influence the rate of heat loss include body mass index, body surface area to volume ratios, clothing and other environmental conditions.
- Physiological changes occur in the cardiovascular system, which manifest in the Osborn J wave and other dysrhythmias, reduced CNS electrical activity, cold diuresis, and non-cardiogenic pulmonary oedema.
- Other symptoms include decreased glomerular filtration rates (GFR), and increased preglomerular vasoconstriction, therefore decreased both renal blood flow (RBF) and GFR.
- Since most clinical thermometers do not measure accurately under 34.4 °C (93.9 °F), determining the core temperature accurately requires a special low temperature thermometer. It is recommended for oesophageal measurements to be taken once the patient is intubated.
- In 2005, the American Heart Association recommended at least 30–45 seconds of feeling for a pulse to verify the absence of a pulse before initiating CPR.
- The Osborn J wave is a classical ECG finding of hypothermia, similar to that of an acute ST elevation myocardial infarction.
- If core temperature falls below 28 °C (82 °F), ventricular fibrillation frequently occurs.
- If core temperature falls below 20 °C (68 °F), asystole (cardiac flatline) occurs, i.e. your heart stops beating, indicating death.
- Freezing occurs when a liquid transitions into a solid because its temperature decreased below its freezing point.
a. Crystallisation
https://en.wikipedia.org/wiki/Crystallization

- This process involves the formation of solids due to the organisation of atoms or molecules into a structure known as a crystal.
- Crystallisation occurs in 2 major steps: (1) Nucleation and (2) Crystal Growth. Crystallisation occurs in 2 major steps: (1) Nucleation and (2) crystal growth, which are driven by both thermodynamic and chemical properties.
- Nucleation involves solute molecules or atoms scattering in the solvent and then clustering together in several clumps, before stabilising under the current operating conditions. When the clusters reach a critical size (determined by a number of factors such as temperature, supersaturation, etc.), they became stable nuclei.
- Once nucleation concludes, the defined and periodic arrangement of the atoms or molecules determines the crystal structure, which refers to the relative arrangement of the atoms or molecules rather than the macroscopic properties of the crystal.
- Crystal growth refers to the nuclei's increase in size after landing the critical cluster size. It is a dynamic process that occurs in equilibrium where solute molecules or atoms precipitate out of solution, and dissolve back into solution.
- One of the drivers of crystallisation is supersaturation, because the solubility of a species is an equilibrium process quantified by Ksp.
- When compounds are able to polymorph, they can crystallise into different crystal structures that may be metastable or kinetically stable. Note that each polymorph is a different thermodynamic solid state and crystal polymorphs of the same compound exhibit different physical properties e.g. dissolution rate, shape (angles between facets and facet growth rates), melting point, etc.
- A number of methods can produce crystals such as cooling, evaporation, addition of a second solvent to decrease the solute's solubility (i.e. antisolvent), solvent layering, sublimation, altering the cation or anion.
- In the laboratory, a solid is dissolved in a solution in which it is partially soluble, usually at high temperatures, to produce a supersaturated solution. The heated solution is subsequently filtered to remove any insoluble impurities. After the filtrate is cooled slowly, newly created crystals are then filtered and washed with a solvent they don't dissolve in, but is miscible with the mother liquor. This process is then repeated to maximise the purity known as recrystallisation.
- Crystallisation processes involves the release of heat of fusion, which increases the entropy of the universe. If the molecules within a pure crystal is heated, it disrupts the crystal architecture, which transitions into a liquid. However, the threshold melting temperature differs between each type of crystal.
- Note that
- melting occurs due to the entropy (S) increase in the system by spatial randomisation of the molecules compensating the enthalpy (H) loss due to the crystal packing forces being broken:
- When the molten crystal cools to a threshold temperature beyond the turning point, its molecules reinstates its original crystalline form. This is due to the thermal randomisation of the surroundings that compensates for the loss of entropy caused by the reordering of molecules within the system.
 |
| This is a low-temperature SEM magnification series for a snow crystal. The crystals are captured, stored, and sputter-coated with platinum at cryo-temperatures for imaging. |
- When the crystal initially forms in the absence of other crystals present, primary nucleation is initiated. There are 2 categories of primary nucleation, depending on the conditions.
- Homogenous nucleation isn't affected by solids in any manner, including the walls of the crystalliser vessel and particles of any foreign substance.
- On the other hand, heterogeneous nucleation refers to the rate of nucleation increasing due to the solid particles of foreign substances.
- This refers to nuclei forming triggered by the existing microscopic crystals in the magma. There are 2 types of secondary nucleation; one is caused by fluid shear, whereas the other is caused by collisions between already existing crystals with either a solid surface of the crystalliser or with other crystals themselves.
- Fluid-shear nucleation is caused by liquid travelling rapidly across a crystal, clearing nuclei that would otherwise be fused into a nuclei, hence forming new crystals.
- On the other hand, contact nucleation was found to be the most beneficial, thus most effective method for nucleation. Benefits include:
- Upon the formation of a nucleus, other molecules adjacent to the crystal converge to expand its own dimension in successive layers. The pattern of crystal growth resembles the layers of an onion, where each layer contains the same mass of solute. Note that each layer further away from the centre becomes thinner due to the increasing surface area of the expanding crystal.
- The growth rate refers to the supersaturated solute mass attached to the original nucleus across a period of time, which is expressed in kg/(h*m2), which is a constant specific to the process.
- Growth rate is affected by a number of physical factors, e.g. Reynolds number, pressure, surface tension of the solution, temperature, relative crystal velocity in the solution.
 |
| This graph shows the solubility of the system Na2SO4-H2O. |
- The x-axis is the equilibrium temperature, and the y-axis is the equilibrium concentration (as mass percent of solute in saturated solution). The graph shows sulfate solubility significantly decreases below 32.5 °C.
- Assuming the saturated solution's temperature is 30 °C, when it is cooled to 0 °C, a mass of sulfate precipitates due to its change in solubility from 29% (equilibrium value at 30 °C) to roughly 4.5% (at 0 °C).
 |
| A vertical cooling crystalliser in a beet sugar factory |
An example of a cooling crystalliser is the Swenson-Walker crystalliser, designed by Swenson Co. approximately 1920.
- This method involves the solute concentration being increased above the solubility threshold by evaporation in order to achieve crystallisation.
- A majority of industrial crystallisers are evaporative, e.g. sodium chloride and sucrose units, whose production accounts for more than 50% of the world's production of crystals. e.g. The Oslo
- The most common type of evaporative crystalliser involves forced circulation (FC), which features a pump or an axial flow mixer). The pumping device maintains the crystal slurry in homogeneous suspension throughout the tank. Pump flow needs to be regulated in order to modulate the contact time of the crystal mass with the supersaturated solution.
- The Draft Tube and Baffle (DTB) crystalliser, invented by Richard Chisum Bennett at the end of the 1950s, contains an internal circulator with an axial flow mixer inside that exerts upwards in a draft tube, as well as a settling area in an annulus.
- Inside the setting area, the exhaust solution moves upwards slowly in order for large crystals to settle and return to the main circulation. Meanwhile, fine particles smaller than a certain grain size are separated and then disintegrated by changing temperature, therefore lead to additional supersaturation.
- Also known as undercooling, supercooling is defined as reducing the temperature of a liquid or gas below its freezing point skipping the solid phase.
- If the liquid is cooled without any seed crystal or nucleus around which a crystal structure can form, it remains a liquid at temperatures in which crystal homogeneous nucleation occurs.
- For example, the freezing point of water is 273.15 K (0 °C or 32 °F). Water can be supercooled at standard pressure to around 224.8 K (−48.3 °C/−55 °F) down at its crystal homogeneous nucleation. As long as water is pure and free of nucleation sites, it can be supercooled by reverse osmosis or chemical demineralisation.
- If water is cooled at a rate on the order of 10^6 K/s, it bypasses crystal nucleation and transforms into glass i.e. an amorphous (non-crystalline) solid. Angell estimated its glass transition temperature to be around 136 K (−137 °C/−215 °F).
- Droplets of supercooled water tend to exist in stratus and cumulus clouds, which may crystallise on aircraft wings or on crucial instruments and probes such as pitot tubes.
This occurs during the solidification process because of the compositional solid changes, which leads to a liquid being cooled below the freezing point.
For steady-state growth CSL = C0 and the partition function k = CSL / CLS is assumed to be constant, the minimum thermal gradient necessary to create a stable solid front is:
- Animals, such as fish e.g. water flounder, employ supercooling to avoid being frozen and avert cell damage and death. They achieve this by producing antifreeze protenis (AFPs) that bind to ice crystals to prevent water molecules from binding and expanding the growth of ice.
- Plant species such as the evergreen shrubs Rhododendron ferrugineum and Vaccinium vitis-idaea as well as Abies, Picea and Larix species were identified to demonstrate supercooling in order to prevent ice from appearing within the tissue by ice nucleation. This helps maintain water inside the plant cells in liquid state and prevent it from interacting with extracellular ice thanks to cellular barriers such as lignin, suberin and the cuticle.
- Conventional freezers: Drinks are supercooled and form slush when they are opened.
- Organ preservation
- Delivery of liquid-encapsulated drugs
- Using encapsulated droplets of supercooled liquid metal to repair heat sensitive electronic devices i.e. soldering without heat.
- Cryogenic fuels or oxidisers are supercooled below their boiling point (but not below the melting point). This increases its density, thus increases the fuel tank's capacity without increasing its weight.
- Freezing is virtually exothermic because heat and pressure are emitted as liquid becomes solid. The energy released upon freezing is latent heat, known as the enthalpy of fusion, which is the same amount of energy required to melt the same amount of the solid.
- The only exception to the general rule is supercooled helium, e.g. Helium-3 has a negative enthalpy of fusion at temperatures below 0.3 K, and helium-4 has a slightly negative enthalpy of fusion below 0.8 K.
- Vitrification comes from the Latin vitreum, meaning "glass" via French vitrifier, therefore it is defined as the substance's transformation into a glass, or a non-crystalline amorphous solid.
- In ceramics, the firing process partially fuses claw, or of a body to begin the vitrification procedure. Dodd & Murfin (1994) stated the proportion of glassy bond increases and the apparent porosity of the fired product gradually decreases.
- Examples of pottery made impermeable to water by glazing or by vitrification include bone china, porcelain and sanitary ware.
- The American Society of Testing Materials defined "vitreous" as "being less than 0.5% absorption, except for floor and wall tile and low-voltage electrical insulators, up to 3% water absorption."
- Cooling sucrose slowly to produce crystal sugar (or rock candy), or cooling sucrose rapidly to produce syrupy cotton candy (candy-floss / fairy floss).
- Cryo-electron microscopy: Cooling samples rapidly to prevent damage for imaging with an electron microscope. This invention lead to a 2017 Nobel Prize for chemistry.
- Ordinary soda-lime glass: Used in windows and drinking containers, it is produced by combining silicon dioxide with sodium carbonate and lime (calcium oxide).
- Geomelting: Disposal and long-term storage of nuclear waste or other hazardous wastes. Combination of waste and glass-forming molecules in a furnace to form molten glass, which solidifies in canisters, hence traps the waste. The ultimate form of waste resembles obsidian, therefore a non-leaching, durable material that effectively encases the waste.
- Cryopreservation of human egg cells (oocytes) and embryos, as well as brains (by Alcor) and to the upper body by the Cryonics Institute.
- This branch of biology studies the effects of low temperatures on living things within Earth's cryosphere or in science. The term is derived from the Greek words κρῧος [kryos], "cold", βίος [bios], "life", and λόγος [logos], "word" [science].
- The history of cryobiology dates back to antiquity, as early as 2500 BC in Egypt. Hippocrates recommended the best treatment to stop bleeding and swelling is to cool the affected area.
- In 1949, a team of scientists led by Christopher Polge cryopreserved bull semen for the first time. This popularised the cryopreservation of organs (e.g. hearts), tissues and cells in cold environments.
- In the early 1970s, the development of controlled-rate and slow freezing techniques resulted in the first human embryo frozen birth by Zoe Leyland in 1984.
- In 1986, Dr Christopher Chen reported the world’s first pregnancy using slow-frozen oocytes from a British controlled-rate freezer.
- Organs, cells, tissues or whole specimens are cooled to temperatures between −80 to −196 °C for cryopreservation. This is because for every 10 °C decrease in temperature corresponds with a 50% decrease in oxygen consumption.
- Hyperthermic organs and tissues extracted from hibernating animals for transplantation require additional solutions to counteract acidosis, decreased sodium pump activity, and elevated intracellular calcium. e.g. Viaspan (made in University of Wisconsin), HTK, Celsior. These solutions also contain components to minimise the effects of free radicals, prevent oedema and replenish ATP levels, etc.
- Red blood cells are typically cooled at a rate of around 100 °C per second, whereas stem cells are cooled at a rate of around 1 °C per minute.
- A 2010 report by the Pacific Fertility Centre stated cryopreserved human gametes and two-, four- and eight-cell embryos can survive at -196 °C for 10 years under controlled laboratory conditions.
- Bacteria species such as Carnobacterium pleistocenium, Chryseobacterium greenlandensis, and Herminiimonas glaciei were revived after being frozen in air for millennia. Pseudomonas syringae is known to generate specialised proteins to nucleate ice, allowing its formation on the surface of various fruits and plants at about -2°C. Listeria develop slowly in temperatures as low as -1.5 °C and can persevere in frozen foods for a period of time.
- In 1861, Thomas Sutcliffe Mort established the world's first freezing works at Darling Harbour in Sydney, Australia, which later became the New South Wales Fresh Food and Ice Company.
- In 1885, the first loads to be frozen in insulated cases onboard ships from Russia to London were chickens and geese.
- In 1929, Clarence Birdseye introduced "flash freezing" in USA after his fur-trapping expeditions to Labrador in the 1910s.
- In 1934, the Icelandic Fisheries Commission was founded to initiate innovation in the fisheries industry and promote quick-freezing fishermen's catches.
- Slows decomposition by converting residual moisture into ice, and suppress the growth of most bacterial species. However, this process is not as effective as thermal techniques such as boiling in preserving food because pathogens tend to survive colder environments rather than warmer environments. It is thought the pathogens in frozen food are deactivated rather than killed, hence they may reactivate after the food thaws.
- Retains most nutrient content of frozen food with subtle losses of vitamins such as Vitamins C, B1 (Thiamine), B2 (Riboflavin) and A (Carotene). Note that any vitamin loss is likely due to preparation of food for the freezing process.
- The observation of a hot liquid freezing faster than the same liquid but colder, under similar conditions, is known as the Mpemba effect. It was named after Tanzanian schoolboy Erasto Bartholomeo Mpemba, who published a story of this phenomenon in 1963.
- Early modern scientists and philosophers such as Francis Bacon, René Descartes, Aristotle and Joseph Black described similar observations to the Mpemba effect.
- There is debate regarding the theory behind its mechanism and the parameters responsible for this phenomenon. There is also ambiguity regarding the moment water freezes either in the form of a visible surface layer of ice, the entire volume of water becomes a solid block of ice or the water reaches 0 °C (32 °F). Note that water can still be liquid at 0 °C (32 °F) due to existence of heat it contains.
What is ice?
- Ice is a naturally occurring crystalline inorganic solid with a lattice structure consisting of water molecules. At atmospheric pressure, ice is roughly 8.3% less dense than its liquid water, which is equivalent to a volumetric expansion of 9%.
- Its density is between 0.9167 and 0.9168 g/cm3 at 0 °C and standard atmospheric pressure (101,325 Pa), whereas water has a density of 0.9998–0.999863 g/cm3 at the same temperature and pressure.
- When liquid water is at freezing point, the water molecules strengthen its hydrogen bonds to allow the packing of molecules to be less compact in the solid. Therefore, ice is buoyant on liquid water.
- A 2012 article reported that ice sheets on sea water permeated with brine-filled channels help sustain sympagic organisms such as bacteria, algae, copepods and annelids. This provides a source of food for aquatic species such as krill and specialised fish e.g. the bald notothen. These creatures are subsequently hunted down by larger animals such as emperor penguins and minke whales.
- For ice to melt, it needs to absorb as much energy as heat to increase the temperature of an equivalent mass of water by 80 °C due to the strength of the intermolecular hydrogen bonds. The amount of energy required to break those hydrogen bonds as ice melts into liquid water is known as its heat (enthalpy) of fusion, which is about 333.55 J/g.
- Ice absorbs more red light due to an overtone of an oxygen–hydrogen (O–H) bond stretch, hence it appears blue-greenish.
 |
| This is a 3D crystal structure of H2O ice Ih (c) composed of bases of H2O ice molecules (b) located on lattice points within the 2D hexagonal space lattice (a). |
 |
| This graph shows the linear relationship between pressure and temperature for melting ice. |
- There are about 20 known solid crystalline phases of water, as well as an amorphous solid state at various densities.
- When pressure increases, a majority of liquids freeze at higher temperatures because the pressure binds the molecules together. Due to the water's strong hydrogen bonds, pressures greater than 1 atm (0.10 MPa) would make water freeze at temperatures below 0 °C.
- Murphy (2005) stated that ice, water and water vapour can coexist at the triple point, which is exactly 273.16 K (0.01 °C) at a pressure of 611.657 Pa. Note that in May 2019, the definition of the Kelvin was 1/273.16 of the difference between this triple point and absolute zero. Iglev et al. (2006) demonstrated ice was challenging to superheat and recorded its temperature increased from −3 °C to 17 °C for about 250 picoseconds.
- La Placa et al. (1972) found at least 15 ice phases (except ice X) can be recovered at ambient pressure and low temperature in a metastable form. Each type is distinguished by their crystalline structure, proton ordering, and density.
 |
| This is a water phase diagram being extended to negative pressures calculated with TIP4P/2005 model. |
 |
| Semi-logarithmic/plot graph of (Log-lin) pressure-temperature phase diagram of water. The Roman numerals correspond to several ice phases listed below. |
 |
| Carl David's (2016) suggested formulation of the phase diagram for certain ices and other phases of water. |
- Also known as non-crystalline or "vitreous" ice, it is an amorphous solid form of water that lacks the long-range order in its molecular arrangement. They form either by rapid cooling of liquid water or compressing ordinary ice at low temperatures.
- To make amorphous ice, liquid water is cooled to its glass transition temperature (about 136 K or −137 °C) in milliseconds in order to prevent the spontaneous nucleation of crystals. Factors that can influence the production of amorphous ice include pressure and the existence of cryoprotectants.
- Formed in the laboratory by a slow accumulation of water vapour molecules onto a smooth metal crystal surface under 120 K.
- LDA is more viscous than normal water when it melts past its glass transition temperature (Tg) between 120 and 140 K. It has a density of 0.94 g/cm3, which is denser than ordinary ice.
- This forms when ice Ih is compressed at temperatures below ~140 K. At 77 K, HDA forms from ordinary natural ice at around 1.6 GigaPascals (GPa) and from LDA at around 0.5 GPa, which has a density of 1.17 g/cm3.
- Discovered by Osamu Mishima in 1996, VHDA forms as its temperature increases to 160 K at pressures between 1 and 2 GPa and has a density of 1.26 g/cm3 at ambient pressure and temperature of 77 K.
- Amorphous ice typically appear in the Earth's summer polar mesosphere, where noctilucent clouds situate. These extremely low temperature are often detected in outer space environments such as molecular clouds, circumstellar disks and the surface of objects in the outer solar system.
- Studies found amorphous ice can form from crystalline ice when its structure is damaged by irradiation from ultraviolet photons, or high-energy electrons and ions at temperatures less than 77 K.
- On the near-infrared spectrum, amorphous ice has distinct 1.65 μm water absorption line, which are influenced by ice temperature and crystal order. This helps it distinguish from crystalline ice, which has 3.1 μm water absorption line.
 |
| This diagram shows the phase space of ice Ih with respect to other ice phases. |
- Also known as ice-phase-one, it is the hexagonal crystal form of ordinary ice, which is typically in the biosphere. Its density is about 0.917 g/cm3, thanks to the hydrogen bonds that spreads the atoms apart in the solid phase. This allows it to float on water, unlike other solid materials.
- When it cools to about −211 °C (62 K; −348 °F), its density increases. As it continues to cool, it then expands again, known as negative thermal expansion. Its latent heat of melting is 5987 J/mol, and its latent heat of sublimation is 50911 J/mol.
- Its structure consists of planes of tessellating hexagonal rings, with an oxygen atom on each vertex, and the edges of the rings formed by hydrogen bonds. They alternate in an ABAB pattern, with B plane and A plane being reflections of each other along the same axes as the planes themselves.
- The oxygen atoms along each bond are about 270 picometers apart, and the lattice between bonds in the crystal lattice is roughly 109.5° (tetrahedral angle).
- This form of ice is a metastable cubic crystalline, which was discovered by Hans König in 1943. Its oxygen atoms are organised in a diamond structure similar to Ih.
- Studies stated it occurs at at temperatures between 130 and 220 K (-143 and -53°C) upon cooling, and exists up to 240 K (−33 °C) upon warming, the threshold temperature to transition to Ih. A number of studies in the 1960s found Ic forms from supercooled water as well as amorphous ice and ice variants II, III and IV.
- Whalley (1981) claimed ice Ic can form in the upper atmosphere and associate with the creation of Scheiner's halo, around 28 degrees from the Sun or the Moon.
- First described and recorded by Gustav Heinrich Johann Apollan Tammann in 1900, ice II is a rhombohedral crystalline form of ice. It is formed by compressing ice Ih at temperature of 190–210 K.
- This form of crystalline has a metastable rhombohedral structure, which is created by heating high-density amorphous ice slowly at a pressure of 810 MPa.
- This monoclinic crystalline phase of water is formed by cooling water to 253 K at 500 MPa. Ice V has a density of about 1.24 g cm3 (at 350 MPa).
- Its structure includes 4-membered, 5-membered, 6-membered, and 8-membered rings, with a sum of 28 molecules in the unit cell.
- Discovered by P.W. Bridgman in January 1912, this variant of ice forms at high pressure at about 1 GPa (= 10 000 bar) and temperatures ranging from 130 up to 355 Kelvin (−143°C up to 82°C).
- Its density is 1.31 g/cm³, and it has a tetragonal crystal system with the space group P 42/nmc. Its unit cell consists of 10 water molecules and its dimensions include a = 6.27 Å and c = 5.79 Å.
- This cubic crystalline variant of ice is formed by cooling liquid water to room temperature above 3 GPa (30,000 atmospheres) or decompressing heavy water (D2O) ice VI below 95 K.
- The structure consists of a hydrogen bond framework that includes 2 interpenetrating (but non-bonded) sublattices.
- Its density is about 1.65 g cm-3 (at 2.5 GPa and 25 °C (77 °F; 298 K)). The cubic unit cell has a side length of 3.3501 Å (for D2O, at 2.6 GPa and 22 °C (72 °F; 295 K)), which consists of 2 water molecules.
- This variant of ice is stable at temperatures below 140 K and pressures between 200 and 400 MPa. It has a tetragonal crystal lattice and a density of 1.16 g/cm³.
- This is a proton-ordered symmetric ice that forms at about 70 GPa of pressure.
 |
| This diagram is a crystal structure of Ice XI viewed along the c-axis. |
- It is the hydrogen-ordered form of Ih that contains an orthorhombic structure with space group Cmc21, which consists of 8 molecules per unit cell. Its lattice parameters at 5 Kelvin include a = 4.465(3) Å, b = 7.859(4) Å, and c = 7.292(2) Å. It was first identified by Shuji Kawada and others in 1972 by experiments.
- Researchers suggested ice XI could form at low pressures at temperatures at 50 - 70 K in locations of the outer space system or within permanently shaded polar craters on the Moon and Mercury, and Jupiter and Saturn as well as the upper atmospheres of Uranus, Neptune, Pluto and Charon.
- First discovered in 1996 by Lobban, Finney & Kuhs, this variant of ice is a metastable, dense, crystalline phase of solid water. Researchers cooled liquid water to 260 K (−13 °C; 8 °F) at a pressure of 0.55 gigapascals (5,400 atm) to obtain Ice XII. Other methods of producing Ice XII are rapidly compressing ice Ih at 77 K (−196.2 °C; −321.1 °F) or by warming high density amorphous ice at pressures between 0.8 to 1.6 gigapascals (7,900 to 15,800 atm).
- It exists as a tetragonal, metastable, dense crystalline form of ice, which has a topological structure of seven- and eight-membered rings, a 4-connected net (4-coordinate sphere packing).
- This monoclinic crystalline phase forms when water is cooled to under 130 K at 500 MPa. It is the proton-ordered form of ice V.
- This orthorhombic crystalline form is created when water is cooled below 118 K at 1.2 GPa. It is the proton-ordered form of ice XII.
- This variant of crystalline ice is a proton-ordered form that is produced by cooling water to around 130 K at 1 GPa (9820 atm). In 2009, Christoph Salzmann at the University of Oxford stated one of the properties of ice XV is its antiferroelectricity.
- This crystalline form of ice has the smallest density at 0.81 g/cm3, which is topologically equivalent to the empty structure of clathrate hydrates. Falenty, Hansen & Kuhs (2014) achieved this by extracting gas molecules from a neon clathrate under vacuum at temperatures below 147 K.
- Ice XVI is thermodynamically unstable at the experimental conditions, therefore it needs to be preserved at cryogenic temperatures.
- When ice is squeezed between 2 layers of graphene, square ice forms at room temperature. Discovered in 2014, it is known that water vapour and liquid water could permeate laminated sheets of graphene oxide. A 2015 study suggested van der Waals forces may be responsible for the formation of square ice at more than 10,000 atmospheres of pressure.
- Known as superionic water / ice, its oxygen ions develop a crystalline structure and its hydrogen ions move freely. When water molecules separate, the oxygen ions crystallise into an uniformly spaced lattice and the hydrogen ions float around freely within the oxygen lattice. The mobile hydrogen ions gives the superionic water its high conductivity, making it a superionic conductor.
- At pressures above 50 GPa (7,300,000 psi), researchers hypothesised that superionic ice would exist as a body-centered cubic structure. A 2013 study suggested superionic ice would become a more stable face-centered cubic lattice at pressures in excess of 100 GPa (15,000,000 psi).
- Charlie Osolin (2010) conjectured that Uranus and Neptune contains a layer of superionic water, however they may be other elements beneath the surface of ice giant planets that prevent the creation of superionic water.
- A proton-ordered form of ice VI produced from water being cooled to around 100 K at around 2 GPa.
- Ice has a low coefficient of friction due to the pressure of the object interacting with the ice, which melts a thin ice layer and allows the object to glide across the surface. For instance, the blade on the ice skate exerts pressure on the ice melts a thin ice layer and lubricates the layer between the ice and the blade.
- Makkonen & Tikanmäki (2014) theorised the melting of a thin ice layer due to the frictional heating is the primary factor behind ice's slippery nature. However, more research is required to understand the physical mechanisms behind the frictional properties of ice.
Where does ice naturally form?
- On the ocean e.g. Sea ice
- On land and structures e.g. Ice sheet, ice cap, aufeis, freezing rain, ice dam
- On rivers and streams e.g. Ice jam, ice disc, Pancake ice
- On lakes e.g. Shelf ice, candle ice, ice shove
- In the air e.g. Rime, ice pellets, hail, snow, diamond dust
- Around 400 BC, Persian engineers learnt how to store ice in the desert during the summer season. The naturally cooled refrigerators they used to store bulk amounts of ice were called yakhchal (meaning ice storage).
- In 16th and 17th century England, low-lying areas along the Thames Estuary often flooded during the winter. This allowed ice harvest industries to thrive between the seasons, where harvested ice is stored in insulated wooden houses.
- In 1799, the first load of ice was transported from New York City to Charleston, South Carolina. During the early 1800s, harvesting ice subsequently became a popular industry.
- Ice is harvested for ice and snow sculpture events such as the annual Harbin International Ice and Snow Sculpture Festival from locations with abundant amounts of ice such as the frozen surface of the Songhua River.
- On an industrial scale, ice is used for chemical manufacturing, concrete mixing and curing. In the 2006 ASHRAE Handbook, icemakers generate 3 types of fragmentary ice: flake, tubular and plate.
- Ice scrapers are used to break the ice and remove them from the surface of roads and windows, because black ice is difficult to see.
- Cars have defrosters on its rear windows to allow the moisture to dissipate in order to remove a thin layer of ice crystals.
- Spray and freezing rain, and icebergs pose as dangerous hazards to ships in different ways. A famous ship that sunk after hitting an iceberg is the Titanic.
- In wintry and snowy conditions, a layer of ice can form on the wings or control surfaces of an aircraft as it climbs through air layers of varying temperatures and humidity. It disrupts air flow above the wing, which severely hampers its performance in the air.
- Aircraft that contain reciprocating engines with carburetors have carburetor air intake heaters in order to combat the effects of adiabatic cooling, hence remove ice from the carburator and allow air flow through the engines.
This sport involves people gliding across snow-covered terrain on skis. Skiers often carry additional equipment such as boots, bindings, poles, helmets, suits, goggles and gloves. The most common type of skiing performed at ski resorts is alpine skiing, which occurs at a piste.
Also known as sledging or sleighing, this sport involves a person sliding across the snow onboard a sled or sleigh in a prone or seated position. It is the basis of 3 Olympic sports: luge, skeleton and bobsledding. Some people slide across sand on a taboggan in an activity known as sandboarding.
This winter sport involves a person sliding down a snow-covered slope on a snowboard fastened to their feet. This sport includes certain characteristics of other winter sports such as skateboarding, sledding, surfing, and skiing.
This activity involves people riding on a motorised vehicle called a snowmobile or a Ski-Doo, snowmachine, sled, motor sled, motor sledge, skimobile, or snow scooter for winter travel and recreation on snow. This vehicle is designed to function on an open field of snow / ice or a road / trail. They can carry up to 2 people, contain no enclosures besides a windshield, and their engines typically drive a continuous track at the rear.
This winter sport involves two teams of 6 players competing against each other on ice skates in an ice skating rink with delineated markings to score the more points by placing the puck into the goal using ice hockey sticks after a specified amount of game time expires, usually three periods of 20 minutes each. This sport is commonly played in Canada, USA and some European countries, whereas in northern Russia, it's referred to as bandy.
- Cooling and preserving food in iceboxes
- Cooling drinks with ice cubes or crushed ice. When the ice absorbs heat and maintains the drink at around 0 °C (32 °F), it melts.
- In air conditioning systems, battery- or solar-powered fans project hot air over the ice.
- Cold packs contain ice to decrease swelling by slowing down blood flow, as well as pain by pushing it down against a region of the body.
- In 1973, engineers used pack ice to construct Antarctica's first floating ice pier, which served the purpose of cargo operations to load and offload ships.
- Ice can be used to construct ornamental ice sculptures such as igloos, ice castles and ice hotels, but living in them are impractical.
- Makkonen (1994) stated a number of roads a few railroads were paved over iced-over lakes and archipelago lakes such as those in Siberia and Canada.
- Pykrete (wood fibres combined with ice) was studied as part of a WWII Allied programme called Project Habbakuk as a possible material for warships, particularly aircraft carriers, because ice was a viable material to construct a vessel immune to torpedoes, as well as a large deck.
2. Black ice
The USA's National Weather Service defined it as "patchy ice on roadways or other transportation surfaces that cannot easily be seen". It is also described as clear (not white) with the black road surface visible underneath. It is prevalent during the early morning hours in the winter, particularly after snow melt on the roadways refreezes over night when air temperatures decrease below water's freezing point.
3. Clathrate Hydrates
- They are crystalline water-based solids that physically take the appearance of ice. Small non-polar molecules (usually gases) or polar molecules with large hydrophobic moieties are confined inside the lattice of hydrogen bonded, frozen water molecules.
- Common low molecular weight gases such as oxygen, hydrogen, nitrogen, carbon dioxide, methane, hydrogen sulphide, argon, krypton, and xenon, as well as a number of higher hydrocarbons and freons can transform into hydrates at suitable temperatures and pressures.
- They can occur naturally in large amounts on the seabed, in ocean sediments, in deep lake sediments (e.g. Lake Baikal), and in the permafrost regions as well as in gas pipelines. It is hypothesised clathrates exist on outer planets, stars, moons and trans-Neptunian objects such as τ-Tauri and Herbig Ae/Be stars, Euceladus (one of Saturn's moons).
4. Ice caps
5. Ice caves
It is a type of natural cave that has abundant amounts of perennial (year-round) ice. This means a portion of the cave is colder than 0 °C (32 °F) all year round, and water exists in the cave’s cold zone.
6. Ice circles
- Also known as ice discs, ice pans, ice pancakes or ice crepes, this rare phenomenon occurs in slow moving water in cold climates. These thin circular slabs of ice rotate slowly on a body of water's surface.
- Examples of ice circles are found in Scandinavia and North America, as well as England and Wales, Sheyenne River in North Dakota, Lake Katrine, New York on the Esopus Creek, Presumpscot River in Westbrook, Maine, Baxter State Park in northern Maine, Kennebec River in Skowhegan, Maine and Taltson River, Northwest Territories.
- Ice disks develop on the outer bends in a river where the accelerating water exerts 'rotational shear', which snaps off a chunk of ice and rotates it. The rotating disc grinds against surrounding ice, which smooths it into a circle.
- Ice pans are defined as "surface slabs of ice that develop in the centre of a lake or creek. As the water cools towards freezing point, ice crystals become frazil ice and amalgamate into a pan-shaped formation.
A solid precipitation of supercooled large drops of water that forms in environments with air temperatures between 0 °C (32 °F) and −3 °C (27 °F). It is denser and more homogeneous than hard rime, which accumulates on branches and overhead lines.
8. Ice Crystals
They are solid ice with atomic structures of various length scales such as diamond dust, dendritic crystals, hexagonal columns, and hexagonal plates. Ice crystals are generally symmetrical and exhibit a hexagonal pattern. Square ice crystals form at room temperature when ice is compressed between 2 layers of graphene.
9. Ice Dams

10. Depth Hoar
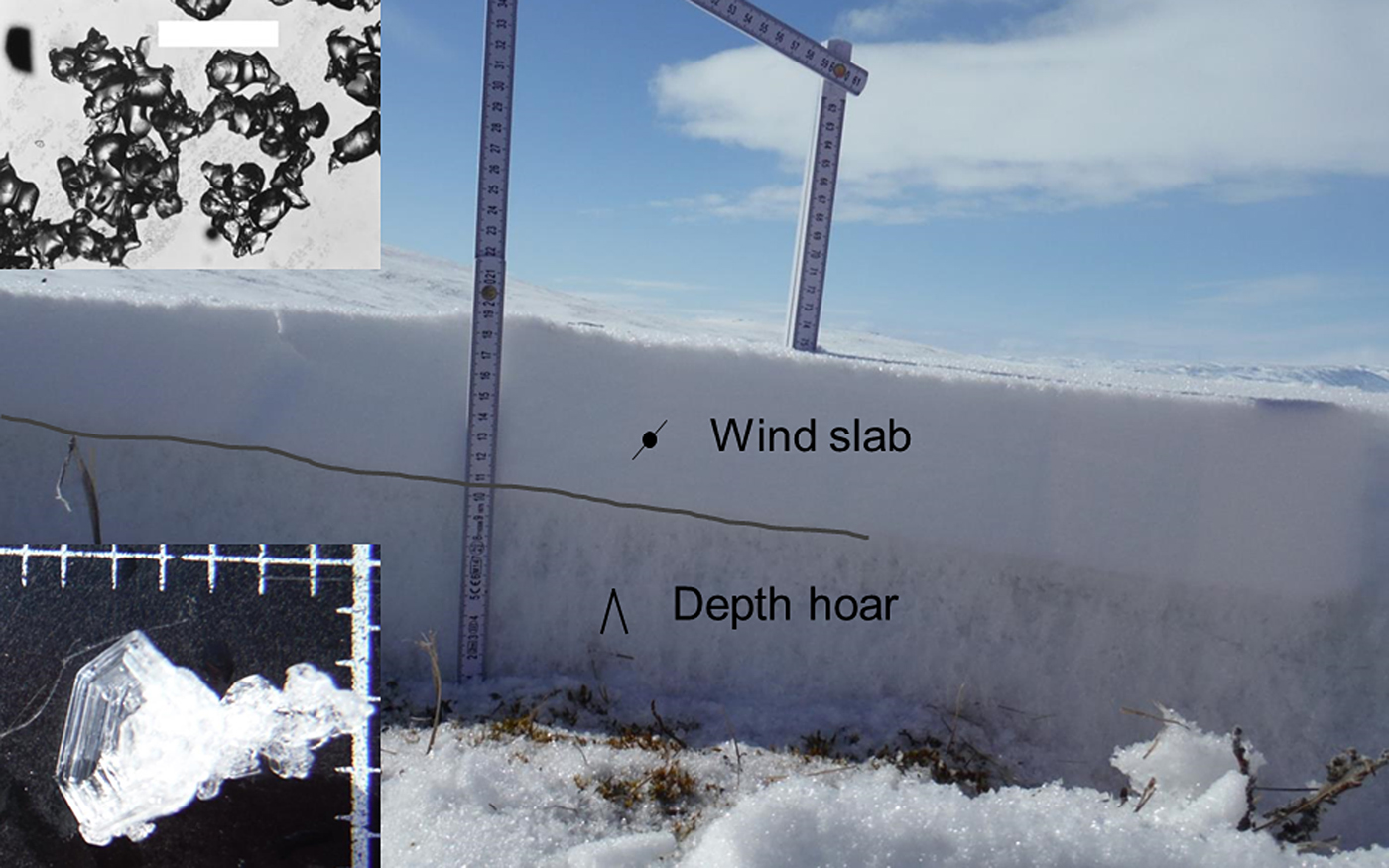
11. Diamond Dust
It is a ground-level cloud consisting of minute ice crystals that is typically found in Antarctica and the Arctic, as well as regions where the temperature is below freezing point. They typically form on the surface due to temperature inversion and the warmer air above the ground blending with the colder air near the surface.
12. Ice Drift
Also known as brash ice, it is defined as sea ice that has detached from the shoreline or any other fixed object (such as shoals, grounded icebergs, etc.). Strong winds and sea currents contribute to it drifting along the water. It consists of individual pieces of sea ice at least 20 metres (66 ft) across called ice floes.
It is a mass of interconnected valley glaciers on a mountain mass with protruding rock ridges or summits. They are observed in colder climates and mountainous regions of the world where precipitation can occur such as Norway, the Alps, Columbia Icefield and the Northern and Southern Patagonian Ice Fields in Chile.
14. Firn
Derived from the Swiss German firn meaning "last year's", cognate with before, it is snow left over from previous seasons and then recrystallised into a substance denser than névé. Its density ranges between 0.4 g/cm³ and 0.83 g/cm³, and it can be located underneath snow that accumulates at the head of a glacier.
15. Ice Fog
This type of fog consists of fine ice crystals drifting in the air, which usually appears in the coldest regions such as interior and northern Alaska. Conditions include 100% humidity as air temperature decreases to below freezing point for air crystals to form.
16. Frazil
Frazil ice consists of plate or discoid ice crystals in supercooled turbulent water, which forms in rivers and lakes located in northern latitudes during the winter. They can block water intakes, particularly on the intake trash rack, which negatively impact hydropower plants, nuclear power facilities, water supply facilities, and vessels navigating in cold waters, hence unexpected shut downs of the facility or collapse of the trash rack.
18. Frost flower
A frost flower is made of thin layers of ice that protrude from long-stemmed plants in autumn or early winter, which curl into "petals" that resemble flowers. Different types of frost flowers include needle ice, frost pillars or frost columns (protrude from pores in the soil), and ice ribbons, rabbit frost or rabbit ice (protrude from linear fissures in plant stems).
19. Glacier
It is an immense body of dense ice that moves under its own weight. When snow accumulates beyond its ablation over centuries, it creates a glacier. The word glacier is a loanword from French, which derives from, via Franco-Provençal, the Vulgar Latin glaciārium, which is derived from the Late Latin glacia, and ultimately Latin glaciēs, meaning "ice".
- A glacier initiates at the head and terminates at its foot, snout, or terminus.
- Ablation zone = A region of net loss in glacier mass.
- Accumulation zone = Glacier's upper part, where accumulation exceeds ablation
- Equilibrium line = A contour between the ablation zone and accumulation zone
- Cirques = A bowl- or amphitheater-shaped depression that remains after a glacier melts.
- They typically move downhill driven by the force of gravity and the internal deformation of ice. They also move through basal sliding, which involves sliding over terrain on which it sits, lubricated by liquid water.
- Fracture zones = The rigid top 50 m (160 ft) of a glacier moves as a single unit over the plastic-flowing lower section.
- Crevasses = Cracks that appear in the fracture zone as the glacier moves through irregular terrain due to 2 or more sections of the glacier having different velocities. Shear forces may widen the crevasse and severely damage the glacier. They can be between 46 m (150 ft) and 300 m (1,000 ft) deep. There are transverse, longitudinal, and marginal crevasses, as well as bergschrunds.
- Friction is a determining factor of the glacier's motion because it acts on the bottom of the glacier more than on the top of the glacier. The average glacial speed is roughly 1 m (3 ft) per day, which can vary depending on the conditions and level of friction.
- Glaciers that accelerate after their usual movement as described as "surges", caused by either damage to the underlying bedrock, pooling of meltwater at the base of the glacier, or accumulation of ice beyond a critical "tipping point". This could increase glacial speed to around 90 m (300 ft) per day.
- If glaciers move faster than one km per year, this triggers glacial earthquakes as powerful as a 6.1 on the Richter scale.
- Ogives (Forbes bands) = Alternating wave crests and valleys that appear as dark and light bands of ice on glacier surfaces, which associates with the seasonal motion of glaciers. They form when ice from an icefall disintegrate, which increase ablation surface area during summer. This results in a swale and space for snow accumulation in the winter, which produces a ridge.
- Moraines = Sometimes described as a glacial till, it is an accumulation of unconsolidated debris, such as regolith and rock, found in glaciated regions that was previously dragged along a glacier or ice sheet. It consists of rounded particles that varies in size from sand to boulders.
- Drumlins = Derived from the Irish word droimnín ("littlest ridge"), it is an elongated hill shaped like an inverted spoon or half-buried egg produced by glacial ice interacting with underlying unconsolidated till or ground moraine. Its heights ranges between 15 and 50 meters, and its length can reach around 1km. The steepest slope facing the direction from which the ice progresses is called the stoss, while the gentlest slope remaining in the ice's direction of movement is called the lee.
- Glacial valleys = Also known as a glacial trough, it is a glaciated valley that is wider, deeper and smoother than a mountain valley, displaying a "U" shaped appearance.
- Truncated spurs = A triangular-shaped cliff produced by glacial valleys with truncated spurs of rock or earth.
- Paternoster lake = Depressions produced by plucking and abrasion that are filled by lakes within glacial valleys.
- Fjord = A glacial valley that moves into a large body of water to create a long, narrow inlet with steep sides or cliffs.
- Hanging valley = Valleys of the tributary glaciers that remain above the main glacier's depression when glaciers recede.
- Arête = A narrow ridge of rock that divides two valleys, created by 2 glaciers eroding parallel U-shaped valleys or 2 glacial cliques eroding headwards towards one another.
- Pyramidal peak = Also known as a glacial horn, it is an angular, pointed mountain peak created by cirque erosion due to multiple glaciers diverging from a central point.
- Roche moutonnées = Also known as a sheepback, it is a rock formation formed by a passing glacier. They may appear elongated, rounded or asymmetrical in shape.
- Alluvial plain = A flat landform formed by the deposition of sediment over time by one or more rivers originating from highland regions, from which alluvial soil develops.
- Kettles = Also known as a kettle lake, kettle hole, or pothole, it is a depression / hole in an outwash plain created by retreating glaciers or draining floodwaters. They are created from blocks of dead ice remaining from retreating glaciers that get surrounded by sediment deposited by meltwater streams due to increased friction.
- Bay mud = When the deposition is in an estuary, it consists of deposits of soft, unconsolidated silty clay, saturated with water.
- Glacial deposits = When a glacier's size truncates below a critical point, it stops flowing and becomes motionless. Meanwhile, meltwater within and under the ice drops stratified alluvial deposits in the forms of columns, clusters and terraces. Kames are glacial deposits shaped as hills or mounds. Eskers are glacial deposits that are long and sinuous.
- Loess deposits = When fine glacial sediments or rock flour is carried by wind gusting over the bare surface and get deposited a considerable distance from the original fluvial deposition site.
These photographs illustrate the rapid retreat of the South Cascade Glacier in Washington documented from 1928 to 2003. Notice how the glacier retreat has accelerated in the modern times. This is evidence of climate change significantly worsening due to human impacts. These shots were captured by USGS U.S. Department of Interior research investigating the last 50 years of glacier change.
20. Glaze
Known as glazed frost, it is smooth, transparent and homogeneous ice wrapped around objects, such as tree branches, after freezing rain or drizzle contacts a surface. It can impact tree branches, power lines, support poles, insulators as well as plants and aircraft.
This form of solid precipitation consists of balls or irregular lumps of ice that typically develop in thunderstorm clouds, as well as parent thunderstorms. For more information on hail, read Natural Disasters Part 4 blog post.
22. Hair ice
Also known as ice wool or frost beard, this type of ice develops on dead wood that resembles fine, silky hair. Researchers discovered them on moist, rotting wood from broadleaf trees when temperatures are slightly below freezing point and the air is relatively humid. Each of the smooth, silky hairs is 0.02 mm (0.0008 in) in diameter and up to 20 cm (8 in) long.
23. Iceberg (Calving)
It is defined as a chunk of freshwater ice more than 15 m long floating freely in open (salt) water after it was separated from a glacier or an ice shelf. It is a partial loan translation from the Dutch word jsberg, literally meaning ice mountain, cognate to Danish isbjerg, German Eisberg, Low Saxon Iesbarg and Swedish isberg.
24. Icicle
A ice spike that develops from water freezing as it trickles down from an object. There are 3 ways for icicles to form:
- The size and shape of an icicle is determined by the surfaces it binds to, the melting water and impurities within the water.
- The growth and width of the icicle is influenced by a number of factors such as air temperature, wind speed, and the water flux into the icicle. An average icicle would be roughly 0.1 mm (0.0039 in) long and about 5 mm (0.20 in) wide.
They form from water permeating a confined space within a structural support or a geological formation, which leads to structural fracture as the water freezes and expands. It can damage a number of natural environments such as rock faces, shorelines, as well as property and the environment.
26. Ice Jam
It features floating river ice accumulating at a natural or man-made feature that blocks ice flowing downstream with the river current.
28. Ice Pellets
Known as sleet in the United States, ice pellets are small, translucent balls of ice formed in a layer of above-freezing air situated between 1,500 and 3,000 meters (5,000 and 10,000 ft) above the ground.
29. Ice Sheet
Known as a continental glacier, it is a bunch of glacial ice that blankets terrain covering more than 50,000 km2 (19,000 sq mi). Current ice sheets are located in Antarctica and Greenland. Historical ice sheets that existed at the Last Glacial Maximum (LGM) include the Laurentide Ice Sheet (North America), the Weichselian ice sheet (northern Europe) and the Patagonian Ice Sheet (southern South America).
30. Ice Shove
31. Ice Spike
Ice spikes usually appear as circular ice candles or polyhedral ice towers, occasionally found in containers of frozen rainwater or tap water.
- When surface water nucleates around irregular surfaces where it contacts the container wall and freezes inward.
- If the c-axis of the crystal isn't vertical, the basal plane intersects the surface along a line perpendicular to the c-axis. This means ice needles would propagate across the surface along this line.
- Simultaneously, an ice curtain develops into the supercooled water along the basal plane.
- As the ice film continues to smother the surface, the crystals amalgamate and fix rigidly in place. The ice sheet continues freezing towards the middle until only a small hole remains unfrozen.
- Subsequently, the remaining water is extracted through the hole and a convex meniscus forms, which produces a bulge higher than the surface of the ice.
- As the meniscus' edges freezes, a small dam is generated which increases the water levels and stretches above the ice dam.
- This step is repeated to create a tube of successive layers of ice until the tip seals over or until all the water is frozen.
32. Stalactites
33. Névé
34. Needle Ice
35. Rime Ice
36. Sea Ice
Covering about 7% of the Earth's surface and about 12% of the world's oceans, sea ice is frozen seawater that floats on the ocean's surface.
37. Slurry Ice
This refrigerant consists of millions of ice "micro-crystals" (typically 0.1 to 1 mm in diameter) that are produced within a solution of water and a freezing point depressant.
38. Slush
It is defined as a slurry mixture of small ice crystals and liquid water. Slush acts like a non-Newtonian fluid since it functions as a solid mass until its inner shear forces increase above a threshold to transform it into a liquid. It is a natural hazard on aircraft runways e.g. Munich air disaster, and roadways.
39. Snow
They are ice crystals that form in the atmosphere, expand, precipitate and accumulate on surfaces, then metamorphose until they melt, slide or sublimate, which completes the frozen crystalline water cycle. See more about snow on Natural Disasters Part 4 blog post.
40. Ice Storm
41. Ice Volcano
This conical mound of ice develops over a terrestrial lake caused by an eruption of water and slush through an ice shelf. Ice volcanoes can be found in a number of locations such as the southern coast of Lake Erie, Lake Ontario, and Lake Michigan, as well as the Swedish town of Ystad.
This frozen natural structure develops over the surfaces of bays, rivers, lakes and seas. They enable animal or human migration over a body of water that was considered impassable by terrestrial animals. An example of an ice bridge linked the island of Öland with mainland Sweden approximately 9000 BC.
This form of sea ice fastens to the coastline, the sea floor along shoals or grounded icebergs. They either form in the sea water or by freezing pieces of drifting ice to the shore or other anchor sites. The dimensions of the fast ice is determined by ice thickness, topography of the sea floor and islands. The fast ice in the Arctic seas can be as deep as 20 m (65.6 ft), whereas the fast ice in the Subarctic seas can be as deep as 10 m (32.8 ft).
Do you remember the classic scene in "A Christmas Story" featuring Flick getting his tongue stuck on a frozen pole and he requires the assistance of the fire department to free him? That phenomenon actually happens in the real world. Your tongue is kept warm and moist with saliva, but freezes once it cools below 0°C. The metal in the pole is 400 times of a conductor of heat than your tongue. This is due to the atoms in metals being tightly packed, meaning it transfers heat energy more readily than your tongue. Furthermore, the metal contains free elections, which increases its conductivity between atoms that absorbs heat in the frozen pole. Therefore, when your tongue contacts the iced metal pole, the metal removes heat from your tongue faster than your body is able to replenish the lost heat biologically through vasodilation and vibration at the tongue. Your tongue rapidly loses moisture, loses heat, hence the temperature of your tongue decreases. This freezes the water inside your pores and surface irregularities on your tongue and the pole. The final outcome is you getting your tongue stuck to the pole, as well as being mocked at by others.
https://en.wikipedia.org/wiki/Refrigeration
- When a space, substance, or system is cooled to decrease and/or maintain a temperature below the ambient temperature, this is known as 'refrigeration'. This involves removing heat energy from a low-temperature reservoir and transferring it to a high-temperature reservoir.
- The work of energy transfer is driven by mechanical methods, as well as heat, magnetism, electricity, laser, etc.
- Examples of devices that utilise refrigeration include household refrigerations, industrial freezers, cryogenics, and air conditioning (AC).
- ~1700 BC = Ruler of Mari in Syria, Zimri-Lim, commanded the construction of the first ice house near the Euphrates.
- ~500 BC = Originating from the Persian word for 'ice pit', the yakhchal was an ancient Persian refrigerator. It was constructed from a mortar resistant to heat transmission, which formed the dome shape.
- ~60 AD = McGee (1988) stated the Hero of Alexandria acquired knowledge of the principle of gas behaviour vs temperature, and the principle of the first thermometer later on.
- 1396 AD = Ice storage warehouses called "Dong-bing-go-tango" (meaning "east ice storage warehouse" in Korean) and Seo-bing-go ("west ice storage warehouse") were built in Han-Yang (currently Seoul, Korea).
- 1593 = The first thermoscope was possibly built by Santorio Santorio or Cornelis Drebbel, before the modern one was constructed by Galileo Galilei.
- 1611 - 1613 = Francesco Sagredo or Santorio Santorio added a numerical scale to the thermoscope.
- 1617 = Giuseppe Biancani published his first clear diagram of thermoscope.
- 1638 = Robert Fludd used air thermometer principle with column of air and liquid water to describe a thermometer with a scale.
- 1650 = Otto von Guerike designed and built the world's first vacuum pump and constructed the world's first ever vacuum known as the Magdeburg hemispheres. This invention disproved Aristotle's long-held supposition that 'Nature abhors a vacuum'.
- 1656 = Robert Boyle and Robert Hooke integrated an air pump into the design of the vacuum pump.
- 1662 = A vacuum pump was used to demonstrate Boyle's law (gas law relating pressure and volume).
- 1665 = Boyle theorised a minimum temperature in his publication New Experiments and Observations touching Cold.
- 1679 = Denis Papin designed the safety valve.
- 1702 = Guillaume Amontons used an air thermometer of his own invention to conduct the first calculation of absolute zero, which is about −240 °C. He hypothesised this point indicated the gas reached zero volume and zero pressure.
- 1714 = Daniel Gabriel Fahrenheit invented the first reliable thermometer, which contained mercury rather than a mixture of alcohol and water.
- 1724 = Fahrenheit proposed his own scale, which had finer scale and greater reproducibility than competitors.
- 1730 = René Antoine Ferchault de Réaumur invented an alcohol thermometer and temperature scale, which was less reliable than Fahrenheit's mercury thermometer.
- 1742 = Anders Celsius proposed a scale with zero at the boiling point and 100 degrees at the freezing point of water. Input from the Swedish academy of science lead to its alteration with the boiling point of water displayed at 100 degrees and the freezing point of water displayed at 0 degrees.
- 1755 = William Cullen used a pump to yield a partial vacuum over a container of diethyl ether, which then boiled, that absorbed heat from the surrounding air.
- 1756 = William Cullen lead the first documented public demonstration of artificial refrigeration.
- 1782 = Antoine Lavoisier and Pierre-Simon Laplace invented the ice-calorimeter.
- 1784 = Gaspard Monge liquefied the first gas, which yielded liquid sulfur dioxide.
- 1787 = Charles's law (Gas law, relating volume and temperature) was published to describe the expansion of gases in warmer environments.
- 1802 = John Dalton described "the reducibility of all elastic fluids of whatever kind, into liquids". Gay-Lussac's law or Amonton's law was formulated, which stated the pressure of a given mass of gas varies directly with the absolute temperature of the gas when the volume is kept constant.
- 1803 = Thomas Moore of Baltimore received a patent on refrigeration.
- 1805 = Oliver Evans constructed the first closed circuit refrigeration machine based on the vapour-compression refrigeration cycle.
- 1809 = Jacob Perkins patented the first refrigerating machine.
- 1810 = John Leslie used an air pump to freeze water to ice.
- 1812 = Avogadro's law was hypothesised by Amedeo Avogadro, which stated that 2 given samples of an ideal gas, of the same volume and at the same temperature and pressure, contain the same number of molecules.
- 1823 = Michael Faraday liquified ammonia to cause cooling.
- 1824 = The Carnot Cycle was first proposed by French physicist Sadi Carnot.
- 1834 = Émile Clapeyron first proposed the ideal gas law, and characterised phase transitions between 2 phases in form of Clausius–Clapeyron relation. Meanwhile Jacob Perkins obtained the first patent for a vapour-compression refrigeration system, and Jean-Charles Peltier discovered the Peltier effect.
- 1844 = Charles Piazzi Smyth proposed the concept of comfort cooling.
- ~1850 = Michael Faraday hypothesised that freezing substances increases their dielectric constant.
- 1851 = American scientist John Gorrie patented his mechanical refrigeration machine to make ice to cool the air.
- 1852 = James Prescott Joule and William Thomson, 1st Baron Kelvin discovered the Joule–Thomson effect.
- 1856 = Scottish-Australian James Harrison patented an ether liquid-vapour compression refrigeration system and developed the first practical ice-making and refrigeration room for use in the brewing and meat-packing industries of Geelong, Victoria. August Krönig developed the simplistic foundation of kinetic theory of gases.
- 1857 = Rudolf Clausius suggested a sophisticated theory of gases including all degrees of freedom, as well derives Clausius–Clapeyron relation from basic principles. Carl Wilhelm Siemens proposed the Siemens cycle, which is a technique used to cool or liquefy gases.
- 1858 = Julius Plücker observed for the first time a certain pumping effect due to electrical discharge.
- 1859 = James Clerk Maxwell determined the distribution of velocities and kinetic energies in a gas, and explained emergent property of temperature and heat. This lead to the first law of statistical mechanics. Ferdinand Carré used gaseous ammonia dissolved in water produce the first gas absorption refrigeration system
- 1862 = Alexander Carnegie Kirk invented the Air cycle machine, which was the refrigeration unit of the environmental control system (ECS) in pressurized gas turbine-powered aircraft.
- 1864 = Charles Tellier patented a refrigeration system that used dimethyl ether, a type of refrigerant.
- 1867 = Thaddeus S. C. Lowe patented a refrigeration system that used carbon dioxide. Furthermore, he bought a steamship and installed a compressor based refrigeration device in order to transport frozen meat.
- 1869 = Lowe made an ice making machine using dry carbon dioxide, and Charles Tellier installed a cold storage plant in France. Meanwhile, Thomas Andrews discovered the critical point in fluids.
- 1871 = Carl von Linde built his first ammonia compression machine.
- ~1873 = Van der Waals published and proposed a real gas model, which was named later as the Van der Waals equation.
- 1875 = Raoul Pictet developed a refrigeration machine that used sulphur dioxide to overcome high-pressure issues of ammonia in tropical climates.
- 1876 = Carl von Linde patented equipment to liquefy air using the Joule Thomson expansion process and regenerative cooling.
- 1877 = Whilst they worked independently, Raoul Pictet and Louis Paul Cailletet develop two methods to liquefy oxygen.
- 1879 = Bell-Coleman machine, also known as the Reverse Brayton cycle, is used in jet aircraft for air conditioning systems using bleed air tapped from the engine compressors.
- 1882 = William Soltau Davidson installed a compression refrigeration unit to the New Zealand vessel Dunedin.
- 1883 = Zygmunt Wróblewski condensed experimentally useful volumes of liquid oxygen.
- 1885 = Zygmunt Wróblewski published the critical temperature of Hydrogen as 33 K; critical pressure, 13.3 atmospheres; and boiling point, 23 (K = Kelvin).
- 1888 = Loftus Perkins developed the "Arktos" cold chamber that can preserve food, thanks to an early ammonia absorption system.
- 1892 = James Dewar invented the vacuum-insulated, silver-plated glass Dewar flask, also known as a vacuum flask, which helps maintain the contents' temperature for longer periods relative to its surroundings.
- 1895 = Carl von Linde filed for patent protection of the Hampson–Linde cycle for liquefaction of atmospheric air or other gases.
- 1898 = James Dewar used regenerative cooling his vacuum flask to condense liquid hydrogen.
- 1905 = Carl von Linde successfully obtained pure liquid oxygen and nitrogen.
- 1906 = Willis Carrier patented the basis for modern air conditioning.
- 1908 = Heike Kamerlingh Onnes was the first to liquefy helium.
- 1911 = Heike Kamerlingh Onnes published his research on metallic low-temperature phenomenon characterised by no electrical resistance, which he labelled it 'superconductivity'.
- 1915 = Wolfgang Gaede invented the diffusion pump, which uses a rapid stream of vapour to direct gas molecules in the pump throat down into the bottom of the pump and out the exhaust.
- 1920 = Edmund Copeland and Harry Edwards were the first to use iso-butane in small refrigerators.
- 1922 = Baltzar von Platen and Carl Munters invented the 3 fluids absorption chiller, which are exclusively driven by heat.
- 1924 = Fernand Holweck invented the Holweck pump, also known as a molecular drag pump, which is a type of vacuum pump that uses the drag of air molecules against a rotating surface.
- 1926 = Albert Einstein and Leó Szilárd invented the Einstein-Szilárd refrigerator, which is an absorption refrigerator that lacks moving parts, functions at constant pressure, and requires only a heat source to operate. Meanwhile, the General Electric Company introduced the first hermetic compressor refrigerator.
- 1929 = Canadian David Forbes Keith received a patent for the Icy Ball, which assisted 100,000s of families through the Dirty Thirties.
- 1933 = William Giauque and others created the Adiabatic demagnetisation refrigeration.
- 1937 = Pyotr Leonidovich Kapitsa, John F. Allen, and Don Misener cooled helium-4 to 2.2 K and discovered superfluids. Frans Michel Penning invented a type of cold cathode vacuum gauge, known as the Penning gauge.
- 1944 = Manne Siegbahn invented the Siegbahn pump, which is a type of vacuum pump that obtains and maintains a high vacuum.
- 1949 = S.G. Sydoriak, E.R. Grilly, E.F. Hammel made the first measurements on pure Helium-3 in the 1 K range.
- 1951 = Heinz London invented the principle of the dilution refrigerator, using Helium-3 / Helium-4.
- 1955 = Willi Becker invented the turbomolecular pump, based on the older molecular drag pumps.
- 1956 = G.K. Walters, W.M. Fairbank discovered phase phase separation in Helium-3 / Helium-4 combinations.
- 1957 = Lewis D. Hall, Robert L. Jepsen and John C. Helmer invented the ion pump based on the concept of Penning discharge. This ion pump sputters a metal getter and operates under low pressures.
- 1959 = Aleksandr Petrovich Klimenko invented the Kleemenko cycle, which was a single-stream mixed-refrigerant technique used to cool or liquefy gases.
- 1965 = D.O. Edwards, and others discovered finite solubility of Helium-3 in Helium-4 solution at 0 K.
- 1966 = H.E. Hall, P.J. Ford, K. Thomson invented the concept of the continuous dilution refrigerator.
- 1972 = David Lee, Robert Coleman Richardson and Douglas Osheroff discovered the superfluidity in Helium-3 at 0.002 K.
- 1973 = The linear compressor, powered by a piston engine, was invented, which featured a piston rolling along a linear track to minimise friction and decrease energy loss during conversion of motion.
- 1978 = The groups of Wineland and Dehmelt demonstrated the concept of laser cooling, which constitute a number of techniques involving atomic and molecular samples being cooled down to near absolute zero.
- 1983 = Mikulin, Tarasov, and Shkrebyonock invented the orifice-type pulse tube refrigerator.
- 1986 = Karl Alexander Müller and J. Georg Bednorz discovered high-temperature superconductivity, which is defined as materials behaving as superconductors at temperatures above 77 K, (−196.2 °C; −321.1 °F), the boiling point of liquid nitrogen.
- 1995 = Eric Cornell and Carl Wieman cooled a dilute gas of Rubidium-87 to produce the first Bose–Einstein condensate. This helped them win the 2001 Nobel Prize for Physics.
- 1999 = D.J. Cousins and others used a dilution refrigerator to record an extremely low temperature of 1.75 mK. A new world record was set at 100 picokelvins (pK) by cooling the nuclear spins in a piece of rhodium metal, which remains unbroken today.
- 2000 = The Helsinki University of Technology's Low Temperature Lab in Espoo, Finland reported nuclear spin temperatures below 100 pK. Note that was the temperature of one particular degree of freedom, rather than overall average thermodynamic temperature for all possible degrees in freedom.
- 2014 = In the CUORE collaboration at the Laboratori Nazionali del Gran Sasso in Italy, a copper vessel with a volume of one cubic meter was cooled to 0.006 Kelvins (−273.144 °C; −459.659 °F) for 15 days.
- 2015 = At Massachusetts Institute of Technology (MIT), a container of sodium potassium was cooled to a temperature of 500 nanokelvins.
- 2017 = At the Cold Atom Laboratory (CAL) in the International Space Station (ISS), up to 20 seconds interaction times and as low as 1 picokelvin temperatures are projected to be achievable. This is the same laboratory that created the Bose-Einstein condensate.
- Before 1830, a number of Americans used ice to refrigerate foods because ice-storehouses and iceboxes hadn't been invented yet. Although ice-storehouses and iceboxes became abundant, the use of axes and saws to harvest ice made it arduous and dangerous.
- By the early 1830s, ice became a mass-market commodity that was sold quite cheaply at half of a cent per pound, which significantly increased ice consumption in multiple American cities such as New York City and Boston. It resulted in the formation of a “cooling culture” to preserve dairy products, fish, meat, and even fruits and vegetables in iceboxes.
- In 1755, Scottish professor William Cullen designed a small refrigerating machine that contained a pump to generate a partial vacuum over a container of diethyl ether that subsequently boiled, which absorbed heat from the surrounding air.
- In 1758, Benjamin Franklin and John Hadley investigated the principle of evaporation as a means to rapidly cool an object at Cambridge University, England. They verified evaporation of highly volatile liquids can cool an object past the freezing point of water. After an object cools past the freezing point of water 0 °C (32 °F), a thin layer of ice forms.
- In 1805, American inventor Oliver Evans studied the closed vapour-compression refrigeration cycle in relation to the production of ice by ether under vacuum.
- In 1820, English scientist Michael Faraday liquefied ammonia and other gases by increasing its pressure and decreasing its temperature.
- In 1834, Jacob Perkins constructed the world's first working vapour-compression refrigeration system.
- In 1842, American physician John Gorrie stated the notion that cooling the air for comfort in homes and hospitals can prevent spread of diseases and alleviate mental and physical degeneration. However, his working prototype of a refrigeration system wasn't a commercial success.
- In 1856, British journalist James Harrison constructed the first practical vapour-compression refrigeration system that used either ether, alcohol, or ammonia. He also constructed a mechanical ice-making machine in 1851 and his first commercial ice-making machine in 1854. In the 1860s, he introduced commercial vapour-compression refrigeration to breweries and meat-packing houses.
- In 1859, Ferdinand Carré built the first gas absorption refrigeration system, which utilised gaseous ammonia dissolved in water i.e. aqua ammonia.
- In the 1860s and 1870s, Carl von Linde conducted research on refrigeration due to the demand from brewers for a technology that enabled year-round, large-scale production of lager. His patent allowed the use of gaseous refrigerants such as ammonia, sulfur dioxide and methyl chloride that became popular until the 1920s.
- John Gorrie's 1842 invention of a system that refrigerated water into ice sparked inspiration in scientists and inventors worldwide such as Ferdinand Carre (France) and Andrew Muhl (USA). This lead to the developments of ice-making machines to serve a number of food and beverage industries.
- However, by the turn of the 20th century, pollution and sewage leaked into natural ice, causing a number of health problems in the suburbs such as the outbreak of typhoid fever. This lead to ice harvesting being banned in several US states.
- In the 1840s, refrigerated railroad cars were introduced in the US to transport dairy products, as well as harvested ice across the country.
- In 1881, Dunedin was the first ship to be refitted with a compression refrigeration unit for meat shipment. In the following year, its journey to London from New Zealand became the first first commercially successful refrigerated shipping voyage, which set the foundation of the refrigerated meat industry.
- Subsequent ships such as Marlborough (sister ship to Dunedin), rival New Zealand Shipping Company vessel Mataurua, SS Selembria, and German Steamer Marsala were converted and joined the refrigerated good trade.
- From the 1890s and into the early 20th century, refrigeration became an integral principle in the preservation of food in the meat-packing industries, e.g. Armour, Swift, and Wilson.
- By the mid-20th century, refrigeration units were installed onto trucks or lorries for transport of perishable goods, such as frozen foods, fruit and vegetables, and temperature-sensitive chemicals.
- Refrigeration units haven't shifted into the home yet because of its sheer weight, large size, significant cost to produce, purchase, and maintain, and its potential safety risks such as fire or toxic hazards.
 |
| An early example of the consumerisation of mechanical refrigeration that began in the early 20th century. The refrigerant used in this refrigerator was sulfur dioxide. |
- In 1911, General Electric (GE) was one of the first companies to develop a practical household refrigeration unit, fuelled by gas. This made an electric compressor motor redundant and helped downsize the refrigerator.
- In 1927, GE developed the first electric refrigerator, called the Monitor Top, to cater for customers of electric companies.
- In 1930, Frigidaire produced a synthetic refrigerants based on a chlorofluorocarbon (CFC) chemical that made refrigerators safer and practical for home and consumer use.
- Synthesised by GE, Freon developed smaller, lighter, and cheaper refrigerators that helped increase the ownership of refrigerators in American households to above 50%.
- In the 1970s, the CFC compounds were discovered to be depleting the ozone layer in the atmosphere, which allowed the entry of solar ultraviolet radiation onto Earth's surface. This lead to its ban at the 1987 Montreal Protocol.
- Refrigerated rail cars
 |
| An 1870 refrigerator car. It has hatches in the roof that allows access to the tanks for the storage of harvested ice at each end. |
This rail carriage helped connect the marketplace with the farms, which benefited the beef packing industry. It allowed the distribution of perishable goods such as meat, fruit and vegetables across the USA.
- Expansion from western USA into rural areas.
- Galactic "City"
- During the start of the 20th century, the introduction of refrigeration and evolution of additional technologies lead to the decrease of Americans pursing agriculture on the farms. In 1935, there were 6.8 million farms in the United States and a population of 127 million. Whereas in 2007 US Census, 3.1 million out of 310 million farmers worked on the farms across the country. A 2015 report stated that as US population increased over time, the demand for agricultural products also increased.
- During the 1890s, the frozen meat trade in New Zealand boomed economically, particularly in Canterbury, where 50% of exported sheep carcasses came from in 1900.
- In the USA, the Meat Inspection Act of 1891 was implemented into law due to local butchers and consumers being apprehensive about the quality of the meat sold for consumption.
- On May 11, 1935, US president Franklin D. Roosevelt signed an executive order called the Rural Electrification Administration (REA), an agency that distributed loans to fund electric infrastructure in the rural areas in the aftermath of the Great Depression. Within a few years after signing, more than 300,000 Americans living in rural areas had power delivered directly to their homes. This significantly improved working conditions on farms, enhanced the safety of food production, and allowed the introduction of refrigeration systems in farming and food distribution processes. This supported food preservation and enhanced the safety of food supplies, which in turn, stimulated the development of perishable commodities.
- In the early 20th century, refrigeration stimulated growth in the sales of fresh produce, meat and dairy products in supermarkets, as well as increased food storage for longer periods of time.
- The introduction of refrigeration accommodated hygienic handling and storage of perishables, which enhanced output growth, consumption, and availability of nutritional produce. A 2004 study estimated this increased dairy consumption by 1.4% and overall protein intake by 1.25% annually in the US after the 1890s.
- Refrigeration made storage of fresh produce and perishables easier, which decreased food waste and spoilage, hence making these ingredients cheaper to purchase at the supermarkets. This correlated with a 5.1% increase in American adult stature through improved nutrition. Furthermore, a 2011 study found a correlation between the number of refrigerators per household and decreases in the rate of gastric cancer mortality.
- Air conditioning of private homes and public buildings
- Preserving food in homes, restaurants and large storage warehouses
- Provision of fresh salads to the modern diet year round, enhancing nutrition
- Liquefy gases such as oxygen, nitrogen, propane and methane, for commerce and manufacturing
- Condenses water vapour from compressed air to minimise its moisture content in order to purify compressed air.
- In oil refineries, chemical plants, and petrochemical plants, refrigeration maintains a number of processes at low temperatures such as alkylation of butenes and butane to produce high-octane gasoline.
- Tempering steel and cutlery
- Transportation of temperature-sensitive food and other materials by airplanes, trains, trucks and ships
- Decreases risk of bacterial diseases from consumption of raw seafood
- This method involves cooling a contained area by melting ice, or by sublimating dry ice. A portable cooler achieves non-cyclic refrigeration by pouring ice above the items inside it.
- This method involves removing heat from a low-temperature source and transferred to a high-temperature sink aided by external drive and thermodynamic power cycle. In the power cycle, a high-temperature source provides heat to the engine, which is either converted to work or a low-temperature sink in order to satisfy the second law of thermodynamics.
- Thermodynamic heat pump cycles or refrigeration cycles are conceptual and mathematical models of heat pump, air conditioning and refrigeration system. Heat pumps transmit heat from the source at a lower temperature to the heat sink at a higher temperature.
- The second law of thermodynamics states that heat cannot spontaneously flow from a colder location to a hotter area without any work. e.g. Air conditioner in a room, or a refrigerator.
- Vapour-compression refrigeration system (VCRS) involves a circulating liquid refrigerant absorbing and extracting heat from an area to be cooled and subsequently transfers that heat to another location.
- Circulating refrigerant enters the compressor as a saturated vapour, in which it is compressed to a higher pressure, making it hotter.
- This becomes a superheated vapour that flows across a coil or tubes known, in which it condenses with either cooling water or cooling air.
- In the condenser, the superheated vapour transfers heat from the circulating refrigerant to an external medium, which cools and condenses the gaseous refrigerant into a liquid.
- The condensed (and saturated) liquid refrigerant then flows through an expansion valve where its pressure decreases.
- This leads to an adiabatic flash evaporation of a part of the liquid refrigerant. This auto-refrigeration effect cools the liquid and vapour refrigerant mixture to a temperature colder than the enclosed space to be refrigerated.
- The cooled refrigerant liquid and vapour mixture then flows through the coil or tubes in the evaporator. Air in the enclosed space circulates across the coil or tubes due to either thermal convection or a fan. This air is warmer than the cold liquid refrigerant, which allows heat to be dispersed. This cools the air and evaporates the liquid into a gas whilst it absorbs heat.
- The refrigeration cycle is completed when the refrigerant vapour from the evaporator becomes saturated and flows back into the compressor.
- (1) The circulating refrigerant enters the compressor as a saturated vapour.
- (1 -> 2) The vapour is compressed at constant entropy (isentropically) and leaves the compressor as a superheated vapour.
- (2 -> 3) The vapour flows through the first part of the condenser where it is cooled because its heat has been extracted.
- (3 -> 4) The vapour flows through the other part of the condenser where it condenses into a saturated liquid.
- (4 -> 5) The saturated liquid refrigerant flows through the expansion valve and instantaneously decreases in pressure. This leads to adiabatic flash evaporation and auto-refrigeration of the liquid, which occurs at constant enthalpy (isenthalpic).
- (5 -> 1) The chilled and partially vaporised refrigerant flows through the coil or tubes in the evaporator. In there, the warm air generated by a circulating fan completely vaporises the refrigerant. It occurs at constant pressure and evaporates every drop of liquid after the temperature increases by 4-8 Kelvin in the evaporator. The refrigerant vapour returns to the compressor inlet to complete the thermodynamic cycle.
- Absorption refrigeration involves a heat source (e.g., a flame supplied by fossil fuels, solar energy, waste heat from factories, or district heating systems) is utilised to energise the refrigerator in order to drive the cooling process.
- In 1858, French scientist Ferdinand Carré was the first to develop absorption cooling by using water and sulphuric acid.
- In the early 20th century, there was widespread usage of water-ammonia systems that demonstrated vapour absorption cycle. After the development of of the vapour compression cycle, it lost popularity due to its low COP (~ 1/5 of the vapour compression cycle's COP).
- Commercial production of absorption refrigerators initiated in 1923 by the newly-formed company AB Arctic, which was bought by Electrolux in 1925. In the 1960s, there was a significant demand for absorption refrigerators to be installed in caravans in the USA.
- Common absorption refrigerators utilise refrigerants with relatively low boiling points (less than −18 °C (0 °F)). Compression refrigerators usually contain hydrochlorofluorocarbons (HCFC) or hydrofluorocarbons (HFC), whereas absorption refrigerators usually contain ammonia or water, as well as at least an additional fluid to absorb the coolant, respectively water (for ammonia) or brine (for water).
- Evaporation = A liquid refrigerant evaporates in a low partial pressure environment to remove heat from its surroundings e.g. the refrigerator's compartment).
- Absorption = This fluid extracts the gaseous refrigerant to supply a lower partial pressure. This leads to the creation of a refrigerant-saturated liquid.
- Regeneration = The refrigerant-saturated liquid is subsequently heated, which evaporates it. This occurs at the lower end of a narrow tube, where the bubbles of the refrigerant gas drives the refrigerant-depleted liquid into a higher chamber. From there, gravity forces it to the absorption chamber. The hot gaseous refrigerant traverses a heat exchanger, where it carries heat outside the system to a higher location for condensation. After the refrigerant condenses into a liquid, gravity forces it to the original chamber to drive the evaporation phase.
- This type of refrigeration was developed by MIchael Faraday in 1821, which involves the refrigerant or adsorbate vapour molecules adsorbing onto the solid's surface rather than dissolving into a liquid.
- Refrigerants used in an adsorption system include ammonia, water, or methanol, etc., which experience phase changes between the vapour and liquid states.
- This form of refrigeration uses the Peltier effect to generate a heat flux at the junction of 2 different types of materials. A DC current flow through one material, which transfers heat to the other material. This results in one side of the cooler decreasing in temperature and the other side increase in temperature. The warmer side is linked to a "hot" sink in order to maintain an ambient temperature, while the cooler side drops below room temperature.
Common thermoelectric materials used in semiconductors include bismuth telluride, lead telluride, silicon germanium, and bismuth-antimony alloys.
 |
| Schematic of the basic working principles of magnetic refrigeration |
- This form of refrigeration was first discovered by German physicist Emil Warburg in 1881, followed by French physicist P. Weiss and Swiss physicist A. Piccard in 1917, and the fundamental principles explained by P. Debye (1926) and W. Giauque (1927).
- It uses the magnetocaloric effect (MCE) to cool a material by exposing it to a changing magnetic field.
- Examples of magnetocaloric materials include gadolinium alloy (Gd5Si2Ge2), praseodymium alloy (PrNi5), MnFeP1−xAsx alloys. La(FexSi1−x)13Hx alloys and Ni2Mn-X (X = Ga, Co, In, Al, Sb) Heusler alloys.
2. Isomagnetic enthalpic transfer:

- This form of refrigeration features super elastic materials that change in temperature when it undergoes applied mechanical stress i.e. the elastocaloric effect. If the superelastic material in the austenitic phase is mechanically stressed, it transitions to the martensitic phase by experiencing an exothermic phase transformation, hence heating it up.
- Examples of super elastic materials include shape-memory alloys such as nitinol and Cu-Zn-Al, as well as natural rubber.
- This method theoretically uses a logic gate to operate a refrigerator in an energy efficient manner without violating the laws of thermodynamics. There are 2 energy states in which a particle can exist: ground state and excited state.
- On the fridge's interior, the g state particle absorbs energy from ambient particles, cools them, and itself transitions to the e state.
- On the fridge's exterior, the particles in the e state falls to the g state, instantaneously releases energy and heats the outside particles.
- Finally, the last step involves the power supply driving particles at the e state, as well as inducing an energy-neutral switch when it falls to the g state, completing the cycle.
- Colloquially known as a fridge, this commercial and home appliance comprises of a thermally insulated compartment and a heat pump that transfers heat from the interior to the exterior in order to maintain the cool temperature inside the fridge. It is programmed to maintain the temperature at or below 40 °F (4 °C) and the freezer is regulated at 0 °F (-18 °C).
- It is an essential food storage appliance worldwide because it helps maintain a cool temperature below the optimum temperature range for perishable food storage (3 to 5 °C (37 to 41 °F), which helps decrease the reproduction rate of bacteria, hence reduce the rate of spoilage.
- Early commercial refrigerator and freezer units used gas systems such as ammonia (R-717) or sulfur dioxide (R-764), which posed a safety hazard at home. In 1915, practical household refrigerators were introduced to USA, which gained popularity in the 1930s as it became cheaper and less toxic, and used non-flammable synthetic refrigerants such as Freon-12 (R-12).
- However, it was discovered R-12 damaged the ozone layer, which resulted in its ban in new refrigerators and air-conditioning systems in 1994. Since 1990, modern refrigerators used the less harmful R-134a (tetrafluoroethane) refrigerant.
- The first electric refrigerators for home and domestic use were invented by Fred W. Wolf of Fort Wayne, Indiana in 1913, which contained a unit fixed on top of an ice box. His first device was named the DOMELRE.
- In 1914, engineer Nathaniel B. Wales of Detroit, Michigan proposed an idea for a practical electric refrigeration unit, which inspired the Kelvinator.
- In 1918, William C. Durant bought out a self-contained commercial refrigerator designed by Alfred Mellowes to begin its mass production by the Frigidaire company.
- In 1927, the General Electric "Monitor-Top" refrigerator, designed by Christian Steenstrup, became popular to the public because it resembled the gun turret on the ironclad warship USS Monitor of the 1860s. First sold at $US525 at the time, it was the first all-steel cabinet.
- Absolute zero is defined as the lowest limit of the thermodynamic temperature scale, which is the state at which the enthalpy and entropy of a cooled ideal gas approach their minimum value, denoted as zero Kelvin.
- It's known that the fundamental particles of nature have minimum vibrational motion, retains only quantum mechanical, zero-point energy-induced particle motion.
- By extrapolating the ideal gas law, the theoretical lowest temperature is determined to be -273.15 degrees Celsius (-459.67 degrees Fahrenheit), also known as 'absolute zero Kelvin or Rankine'.
- Robert Boyle was one of the first scientists to propose the idea of an absolute minimal temperature. In his 1665 New Experiments and Observations touching Cold detailed the debate known as the primum frigudum.
- Back in the 17th century, scientists were faced with a difficult question. Is there a limit to the degree of coldness possible?
- In 1702, French physicist Guillaume Amontons first addressed this question by associating with his contributions in the air thermometer. This device signified the current air temperature according to its height at which a certain mass of air sustained a column of mercury i.e. the volume, or "spring" of the air varied with temperature.
- Therefore, he argued that the zero on his thermometer indicated the temperature at which the air's spring decreased to nothing. The scale Amontons used had a demarcation of the water's boiling point at +73 and the ice's melting point at +51.5. Hence, the zero was equivalent to about -240 on the Celsius scale.
- Amontons argued absolute zero is unattainable, thus never attempted to calculate it explicity.
- In 1740, George Martine published the value of -240 degrees Celsius or "431 divisions below the cold of freezing water".
- In 1779, Johann Heinrich Lambert approximated the value for 'absolute cold' as -270 degrees C (- 454 degrees F; 3.15 K).
- After James Prescott Joule's computation of the mechanical equivalent of heat, Lord Kelvin formulated a scale of absolute temperature that was independent of the attributes of any chemical in 1848. Furthermore, Kelvin referenced Carnot's theory of the Motive Power of Heat and data published by Henri Victor Regnault. This lead them to conjecture that -273 degrees C was point zero on the air thermometer.
- However, this value was not immediately accepted, because there were many other estimates ranging from -274.5 degrees C (-462.10 degrees F) to -271.1 degrees C (-455.98 degrees C).
- In 1845, Michael Faraday liquefied most known gases by cooling them to temperatures of -130 degrees C (-202 degrees F; 143 K).
- In 1873, Dutch theoretical scientists Johannes Diderik van der Waals successfully liquefied gases such as oxygen, nitrogen, and hydrogen by increasing the pressure and decreasing the temperature to extreme levels.
- Louis Paul Cailletet and Raoul Pictet successfully decreased the temperature of droplets of liquid air to -195 degrees C (-319 degrees F; 78.1 K).
- In 1983, Polish professors Zygmunt Wróblewski and Karol Olszewski produced liquid oxygen by decreasing oxygen gas to -218 degrees C (-360 degrees F; 55.1 K).
- In 1898, Scottish chemist and physicist James Dewar was the first to liquify hydrogen at -252 degrees C (-421.6 degrees F; 21.1 K).
- In 1908, Dutch physicist Heike Karnerlingh Onnes was the first to liquify helium using precooling stages and the Hampson-Linde cycle to decrease the temperature of helium gas to -269 degrees C (-452.20 degrees F; 4.15 K).
- At temperatures near 0 K (−273.15 °C; −459.67 °F), nearly all molecular motion ceases and ΔS = 0 for any adiabatic process, where S is the entropy. In that case, pure substances can (ideally) produce perfect crystals as T → 0.
- Max Planck's interpretation of the 3rd law of thermodynamics described the entropy of a perfect crystal disappears at absolute zero. The original Nernst heat theorem argued that the entropy change for any isothermal process approaches zero as T → 0:
- According to the Nernst postulate, the isotherm T = 0 coincides with the adiabat S = 0, despite other isotherms being distinct. Since no 2 adiabats intersect, no other adiabat can intersect the T = 0 isotherm. This means no adiabatic process initiated at non-zero temperature can lead to zero temperature.
- When the internal lattice structure extended continually in all directions, this theoretically produces a perfect crystal. The perfect order is reflected by translational symmetry along 3 axes.
- Substances existing in 2 (or more) stable crystalline forms, such as diamond and graphite for carbon, represent the phenomenon of chemical degeneracy.
- According to the Debye model, the specific heat and entropy of a pure crystal are proportional to T3, while the enthalpy and chemical potential are proportional to T4. These values reduce toward their T = 0 limiting values and approach with zero slopes.
- The relationship between changes in Gibbs free energy (G), the enthalpy (H) and the entropy can be represented by the equation: ΔG = ΔH - T*ΔS
- As T decreases, ΔG and ΔH are close to equalling each other (assuming ΔS is bounded). Experiments found that all spontaneous processes (including chemical reactions) eventually have a decreased G as it approaches equilibrium. If the values of ΔS and/or T are small, the condition ΔG < 0 suggests that ΔH < 0, which represents an exothermic reaction.
- When T = 0, the gradients of the derivatives of ΔG and ΔH converge and are equal to zero. This allows ΔG and ΔH to be close in value over a considerable range of temperatures and upholds the approximate empirical Principle of Thomsen and Berthelot.
- In 1995 at the University of Colorado at Boulder NIST-JILA lab, Eric Cornell and Carl Wieman cooled a gas of rubidium atoms to 170 nanokelvins to create the first ever gaseous Bose-Einstein condensate (BEC).
- In 2003, researchers at Massachusetts Institute of Technology (MIT) reached a record cold temperature of 450 ± 80 picokelvins (pK) in a BEC of sodium atoms.
- Certain thermodynamic systems can display negative thermodynamic temperatures such as the Kelvin and Rankine temperature scales. This is not to be confused with negative values on non-thermodynamic Celsius or Fahrenheit scales, which are nevertheless higher than absolute zero.
- In 1949, Lars Onsager first hypothesised the existence of negative temperatures in his analysis of classical point vortices confined to a finite area. Confined point vortices are a system containing a bounded phase space since their canonical momenta are not independent degrees of freedom from their canonical position coordinates. He demonstrated that a system with a bounded phase space experiences peaks in entropy as energy increases.
- If the energy transcends the value where the peak occurs, entropy decreases as energy increases, meaning high-energy states have negative Boltzmann temperature.
- It would seem paradoxical and perplexing that negative temperatures on the Kelvin scale is significantly hotter than any system with positive temperatures.
- In 1956, Norman Ramsay explained that an interaction between a negative-temperature system and a positive-temperature system would lead to heat flow from the negative- to the positive-temperature system. e.g. Population inversion in laser physics.
- A more precise definition of thermodynamic temperature describes the tradeoff between internal energy and entropy contained in the system. On the other hand, the concept of "coldness" is described as the reciprocal of temperature.
- Atkins (2010) stated that systems with positive temperature have higher entropy as additional energy enters the system, whereas systems with negative temperatures have lesser entropy as additional energy enters the system.
- In classical thermodynamics, S is defined in terms of temperature. However, in the function of the possible microstates of the system, S is defined as the statistical entropy, and temperature defines the distribution of energy levels among the possible microstates.
- As temperatures increase, particles transition into higher energy states until the ratio of particles in lower energy states to particles in higher energy states is close to 1.
- Believe it or not, a substance with a negative temperature is not colder than absolute zero, but rather it is hotter than infinite temperature.
- The corresponding inverse temperature scale, for the quantity β = 1/(k*T) (where k is Boltzmann's constant), stretches from low energy to high as +∞, …, 0, …, −∞.
- Since there is no upper bound on the atom's momentum, there is no upper bound to the number of energy states available as additional energy enters the system. This means a negative temperature is not possible to achieve.
- Temperature is dependent on the spread of energy among the translational, vibrational, rotational, electronic, and nuclear modes of a system.
- In certain situations, one or more modes can be isolated to transfer energy with other modes, however the transfer is slower than that within the isolated mode. e.g. Energy flows swiftly among the spin states of interacting atoms, whereas energy transfer between the nuclear spins and other modes is relatively sluggish.
We can then solve for the thermodynamic beta by interpreting it as a central difference without taking the continuum limit:
This proof assumes the microcanonical ensemble with energy fixed and temperature being the emergent property. In the case of a canonical ensemble, the temperature is fixed and energy is the emergent property. This means:
All T increases, the values of Z, E and S also increase, which means they don't enter a negative temperature regime.
 |
| These 3 graphs illustrate entropy, thermodynamic beta, and temperature as a function of the energy for a system of N non-interacting two-level particles. |
- Electronic and nuclear spin systems have a finite number of modes available, which are usually spin up and spin down. Where there is no magnetic field, these spin states correspond to the same energy, or become degenerate. Furthermore, entropy would reach its maximum when half the atoms are in the spin-up state and half are in the spin-down state.
- In the presence of a magnetic field, those spin states will have a different energy level from those that are anti-parallel to it, leading to split energy levels. Moreover, some atoms would align to minimise the system's energy, resulting them being in the lower-energy state.
- When radio waves are fired at the spin system, it may flip the atom's nuclear spin from spin-down to spin-up. Energy input into the system decreases the entropy as the system shifts away from a balanced mixture of spin-up and spin-down atoms, which corresponds to a negative temperature.
- In laser systems, a significant proportion of the system's atoms (for chemical and gas lasers) or electrons (in semiconductor lasers) are in excited states, commonly referred to as a population inversion.
- The requirements for a system in ground state include trace convergence, a meaningful density operator, and a positive semidefinite βH.
- Therefore, if hv < μ, and H is negative semidefinite, that means β must itself be negative, which indicates a negative temperature.
- Braun et al. (2013) used an optical lattice to position upper bounds on the kinetic energy, interaction energy and potential energy of the cold potassium-39 atoms. He adjusted the atoms' interactions from repulsive to attractive with a Feshbach resonance and altered the overall harmonic potential from trapping to anti-trapping, which transformed the Bose-Hubbard Hamiltonian from Ĥ → −Ĥ.
- If this transformation proceeds adiabatically, as long as the atoms maintained in the Mott insulator regime, there is a chance a low entropy positive temperature state would become a low entropy negative temperature state.
- In the negative temperature state, atoms macroscopically occupy the maximum momentum state of the lattice. Furthermore, the negative temperature ensembles equilibrated and demonstrated longevity in an anti-trapping harmonic potential.
What is an ice age?
- The ice age is an extended period of extremely low temperature on Earth's surface and atmosphere, which lead to the formation or expansion of continental and polar ice sheets and alpine glaciers.
- Over the past 3 billion years, it is known about 5 ice ages have occurred on Earth's history. Below is a table of the geological periods the major ice ages are estimated to have occurred.
 |
| Sediment records illustrated the fluctuating sequences of glacials and interglacials during the last several million years. |
- Geological evidence = Found in drumlins, glacial moraines, rock scouring and scratching, valley cutting, and the deposition of till or tillites and glacial erratics. However, this evidence is challenging to date and interpret due to glaciations distorting or eliminating evidence from earlier glaciations.
- Chemical evidence = Ratios of isotopes in fossils present in sediments and sedimentary rocks and ocean sediment cores. A 2004 report stated water consisted of lighter isotopes with a lower heat of evaporation, which decreases with warmer conditions.
- Paleontological evidence = It is theorised, during the glacial period, cold-adapted organisms migrated to lower latitudes, and warm-adapted organisms became extinct or retreated to lower latitudes.
 |
| This graph illustrated the pattern of temperature and ice volume changes associated with recent glacials and interglacials. |
- A glacial period is defined as an interval of time (1000s of years) within an ice age when colder temperatures and advancing glaciers are prominent. They associate with cooler and drier climates throughout a large proportion of Earth, as well as extensive land and sea ice masses from the poles.
- An interglacial period is defined as a geological interval of warmer global average temperature that last 1000s of years, which occur between consecutive glacial periods within an ice age.
- Earth is currently in an interglacial period known as the Holocene that has been occurring for about 11,700 years. There are predictions that the next glacial period would occur in at least 50,000 years from today. Studies suggested elevated greenhouse gas levels lead to anthropogenic forcing, which may outweigh the orbital forcing of the Milankovitch cycles for 100,000s of years.
 |
| This diagram illustrates minimum (interglacial, black) and maximum (glacial, grey) glaciation of the northern hemisphere. |
 |
| This diagram illustrates minimum (interglacial, black) and maximum (glacial, grey) glaciation of the southern hemisphere. |
- A glacial period's positive feedback is influenced by Earth's albedo, which is amount of the sun's energy being reflected off Earth's surface. Earth's albedo increases with ice and snow, but decreases with forests.
- Ewing and Donn (1956) conjectured that an ice-free Arctic Ocean plays a role in increasing snowfall at higher latitudes. In theory, evaporation or sublimation is minimal when there is a sheet of ice over the Arctic Ocean, which results in drier polar regions. This means high-latitude snowfalls can melt during the summer due to low precipitation.
- A lack of oceanic ice pack allows the exchange of waters between the Arctic and the North Atlantic Oceans, increases the temperature of the Arctic Ocean, and decreases the temperature of the North Atlantic.
- During a warming cycle, excess fresh water flows into the North Atlantic would decrease the global ocean water circulation, which would theoretically decrease the temperature in northern Europe, which results in increased snowfall retention during the summer.
- Ice sheets lead to erosion of the land it covers, which decreases the land area above sea level, hence minimise the area for ice sheet to form on.
- Huddart & Stott (2013) stated this attenuates the albedo feedback, as well as sea levels associated with the decreased area of ice sheets.
- Another negative feedback process is aridity increasing with glacial maxima, which decreases the precipitation available to maintain glaciation.
- Nature Geoscience suggested the carbon dioxide emissions generated by human activity would defer the next ice age. Based on evidence regarding Earth's orbit and the historical warm interglacial periods, researchers predicted the next ice age may occur within 1,500 years.
- The Snowball Earth hypothesis suggests severe freezing in the late Proterozoic ceased due to an increase in carbon dioxide levels in the atmosphere, chiefly from volcanoes.
- Despite the evidence of greenhouse gas levels plummeting at the beginning of ice ages during the retreat of the ice sheets, there is difficulty proving its cause and effect.
- Clark et al. (2009) found changes in solar insolation increase the temperature of Earth's surface after an Ice Age, as well as secondary factors such as increases in greenhouse gases.
- According to geological records, ice ages initiate when the continents are positioned to obstruct the flow of warm water from the equator to the poles, which leads to the formation of ice sheets. This means ice sheets increase Earth's albedo, which decrease the absorption of solar radiation.
- Hannah Lee (2014) listed 3 continental positions that contribute to the obstruction of the flow of warm water to the poles.
- When a continent situates over the pole, such as Antarctica.
- When a polar sea is virtually landlocked, e.g. Arctic Ocean.
- When a supercontinent envelops most of the equator, e.g. Rodinia during the Cryogenian period.
- A 1988 study hypothesised the geographic location of the Himalayas may play a role in the current ice age, since it correlated with higher total rainfall and thus decreasing atmospheric carbon dioxide levels, which reduce the greenhouse effect.
- Ocean currents play a role in adjusting the temperature of certain geographic regions, such as the creation of Antarctic ice and a temperate environment in the British Isles.
- In 1996, Svitil suggested the closure of the Isthmus of Panama roughly 3 million years ago resulted in the current glaciation over North America because it obstructed water exchange between the tropical Atlantic and Pacific Oceans.
- During the previous glacial period, the sea-level fluctuated 20–30 m due to sequestered water mainly in the Northern Hemisphere ice sheets. As the collected ice and sea level decreased, water flow through the Bering Strait also decreased, which increased water flow from the North Atlantic.
- This lead to the realignment of the thermohaline circulation in the Atlantic, which elevated heat transport into the Arctic, resulting in melting of polar ice accumulation and thinning of other continental ice sheets.
- Kuhle suggested the ice sheet blanketing the Tibetan Plateau during the Last Glacial Maximum triggered an Ice Age. He thought Tibet's plate-tectonic uplift resulted in changes from bare land to ice with a 70% increase in albedo. This lead to global cooling, which resulted in the Pleistocene Ice Age.
- Studies concluded the combination of decreased Nordic inland ice areas by the weight of the superimposed ice-load and interglacial periods by the 100,000-year cycle of radiation changes result in reoccurring absolute thawing of the inland ice areas.
 |
| This graph shows the past and future of daily average insolation at top of the atmosphere on the day of the summer solstice, at 65 N latitude. |
- The set of cyclic variations in characteristics of Earth's orbit around the Sun is known as the Milankovitch cycles. Each cycle varies in length, which may lead to reinforcement or neutralisation.
- Researchers suggested a possible relationship between Milankovitch cycles and occurrence of glacial and interglacial periods within an ice age. Within the past 400 years, the glacial/interglacial frequencies closely align with the Milanković orbital forcing periods.
- This impacts the Earth's distance relative to the Sun, the angle of Earth's axis, and the rotation around Earth's axis, which redistributes the Earth's area exposed to sunlight. e.g. In July, the amount of solar influx at 65 degrees North latitude varies by as much as 22% (from 450 W/m2 to 550 W/m2).
- During the last 800,000 years, glacial–interglacial oscillations that lasted about 100,000 years correlated with changes in Earth's orbital eccentricity and orbital inclination. Between 3 and 8 million years ago, a period of glaciation that lasted around 41,000 years corresponded with Earth's obliquity or axis tilt. More research is required to fully understand these relationships, and the answer may be associated with resonance in Earth's climate system.
- Muller & McDonald (1997) touched on the fact that Milankovitch's calculations assumed a 2D orbit of Earth. In addition, a 3D orbit of Earth also has a 100,000-year cycle of orbital inclination. They hypothesised a direct relationship between orbital inclination and insolation, as Earth oscillates between known dust bands in the solar system.
- A number of other researchers such as William Ruddiman, Peter Huyber, Peter Ditlevsen and Didier Paillard presented arguments for their own models in order to explain the length of the cycles (26,000 years, 41,000 years, 100,000 years). Proposed models include the modulating effect of eccentricity on precession along with greenhouse gas feedbacks, Earth's 2nd / 3rd climate cycle triggers an ice age, and late Pleistocene glacial cycles transitioning between 3 quasi-stable climate states due to orbital forcing.
- In the distant future, astrophysicists predicted that the Sun's energy output by about 7% every one billion years.
- Sunspot cycles and the Maunder Minimum have occurred during the coldest part of the Little Ice Age. However, there is insufficient evidence to support the theory that the Sun's energy output over a long period of time leads to ice ages.
- A 2016 study hypothesised the Paleocene-Eocene Thermal Maximum was caused by release of methane from clathrates of undersea volcanoes, which amplified greenhouse effect.
- George Rieke (2013) stated that carbon dioxide released from volcanoes associated with periods of high overall temperatures.
- The previous glacial period approximately 8,000 years ago left icy landscapes in Canada, Greenland, northern Eurasia and Antarctica, which included glacial erratics, tills, drumlins, eskers, fjords, kettle lakes, moraines, cirques, pyramidal peaks.
- The ice sheets' weight contributed to the deformation of Earth's crust and mantle, which was restored after the melting of the ice sheets.
- Glaciation associated with reductions in global sea levels, exposure of continental shelves and the creation of bridges between landmasses to enable animal migration.
- Andersens & Borns (1997) suggested the cycle of deglaciation and reglaciation in the Baltic and Scandinavian regions, as well as much of central North America at the end of the last glacial maximum was responsible for the topsy-turvy patterns of land, ice, saltwater and freshwater. Furthermore, the elevation on Scandinavia resulted in the inundation of a vast continental plain that is now North Sea.
- When ice-water is redistributed on Earth's surface, along with the flow of mantle rocks, it affects the Earth's gravitational field and redistributes its moment of inertia, which impacts the angular velocity, axis and the wobble of Earth's rotation.
- Studies in the late 20th century found the weight of the redistributed surface mass filled the lithosphere, which curved it and strained it within Earth.
- Hunt & Malin (1998) stated that deglaciation lead to accelerated slippage of fault lines, which resulted in earthquakes. Moreover, if epicentres situated near the ice margin, it would facilitate ice calving, possibly creating a Heinrich event.


















































































































































