When you haven't eaten for a long period of time, your stomach will begin to rumble or cause discomfort, or your mouth will begin to drool or water. This desire to search for and consume food, or fulfil appetite, is a sensation known as hunger. The term 'hunger' is often referenced in social science and policy discussions to describe the condition of people who suffer from a chronic lack of nutritious food and frequent experience hunger, which result in malnutrition. Kate Ravilious (2005) found healthy, well-nourished people who fast for weeks (between 3 and 10) can still survive.
What is hunger?
Why do you feel pain when you're hungry?
- Known as hunger pangs, they are associated with contractions of your stomach muscles, which are thought to be triggered by increased levels of ghrelin hormone. If blood sugar levels are depleted without being replenished for long periods of time, then ghrelin is released into the bloodstream.
- Hunger pangs can become severe from lack of meals consumed daily (i.e. 1 a day) or eating at inconsistent times over consecutive days.
- Stomach contractions become less powerful with age during the hunger phase, but the secondary effects caused by reduced food intake still occur, which include reduced concentration, irritability, and weakness.
- Carol Kop (2009) stated prolonged insufficient nutrition can increase the susceptibility to disease and hamper the body's ability to heal.
Describe the short-term regulation of hunger and food intake
Short-term regulation of hunger and food intake requires a number of neural signals from the GIT, blood levels of nutrients, GIT hormones, and psychological factors.
i. Neural signals from the GI tract
Vagal nerve fibres from the brain to the gastrointestinal tract (GIT) help evaluate the contents of the gut. Marieb & Marieb (2010) found stretch receptors inhibit appetite upon GIT distention by projection signals along the vagus nerve afferent pathway and inhibiting the hunger centre.
ii. Hormone signals
As food is absorbed into your body, insulin and cholecystokinin (CCK) are released from the GIT to suppress the feeling of hunger. In addition, CCK suppresses hunger by inhibiting neuropeptide Y. Marieb & Marieb (2013) found glucagon and adrenaline levels increase during fasting and hunger, which is subsequently followed by release of ghrelin from the stomach to stimulate appetite.
iii. Psychological factors
2 psychological processes are involved in the regulation short-term food intake, which are 'liking' and 'wanting'.
- Liking = Palatability or taste of the food, which is decreased by frequent consumption.
- Wanting = The desre to consume the food, which is also decreased by frequency consumption of food and possibly due to changes in memory-related processes.
- Thinking about food can interfere with consciousness and be expatiated on as a person watches a commercial or smells a desirable food.
Describe the long-term regulation of hunger and food intake
- In 1994, the hormone leptin was found to be produced by the adipose tissue to provide negative feedback on regulation. Wynne et al. (2015) found leptin is primarily involved in homeostasis and immune responses. When food intake was reduced, leptin levels subsequently reduced in the body, and vice versa.
- Further studies demonstrated appetite regulation is a complex process involved the GIT, numerous hormones, and both the central and autonomic nervous systems.
- Suzuki, Jayasena & Bloom (2011) found appetite can be either stimulated or suppressed by circulating gut hormones that are responsible for the regulation of numerous pathways in the body. For example, cholecystokinin and glucagon-like peptide-1 (GLP-1) suppress appetite, whereas ghrelin stimulates appetite.
i. Effector
- Human appetite is primarily regulated by an organ in the brain called the arcuate nucleus of the hypothalamus.
- Bojanowska & Ciosek (2016) stated dopamine serves as the reward neurotransmitter in appetite.
- Wler et al. (2017) found serotonin functions through the appetite-stimulating neuropeptide Y (NPY)/agouti-related peptide (AgRP) and satiety-inducing proopiomelanocortin (POMC) neurons in the arcuate nucleus.
- Varela & Horvath (2012) found the hormones insulin and leptin suppress appetite through AgRP and POMC neurons.
- The hypothalamocortical and hypothalamolimbic projections allows us to become aware of hunger, and the somatic processes controlled by the hypothalamus includes vagal tone, stimulation of the thyroid (to regulate metabolic rate), the hypothalamic-pituitary-adrenal axis and a number of other mechanisms.
- Wassum et al. (2009) found the palatability of foods is influenced by the opioid receptor-related processes in the nucleus accumbens and ventral pallidum.
- The nucleus accumbens (NAc) coordinates neurotransmitter, opioid and endocannabinoid signals to regulate feeding behaviour.
- The signalling molecules inside the NAc shell that regulate the motivation to eat and the affective reactions for food include acetylcholine (Ach), dopamine (DA), opioids and cannabinoids and their action receptors inside the brain, muscarinic, DA, μ-opioid receptor (MOR) and CB1 receptors respectively.
ii. Sensor
- The hypothalamus senses external stimuli primarily through hormones such as cholecystokini, ghrelin, leptin, orexin and PYY 3-36, all of which regulate the hypothalamic response. Leptin is produced by adipose tissue and the other hormones are produced by the digestive tract.
- Appetite is negatively affected by systemic mediators, such as corticotropin-releasing hormone (CRH), interleukins 1 and 6, and tumour necrosis factor-alpha (TNFα), which explains why sick people tend to eat less food.
- Leptin is secreted exclusively by adipose cells in resposne to increases in body fat mass, which plays an important role in regulating long-term hunger and food intake. One of the important functions is indicating the brain of the body's total energy stores. When leptin levels increase in the bloodstream, they bind to receptors in ARC.
-- Suppress the release of neuropeptide Y (NPY), which subsequently prevents the release of orexins from the lateral hypothalamus. Thus, this reduces appetite and food intake, which contributes to weight loss.
-- Stimulates expression of cocaine and amphetamine regulated transcript (CART)
-- Stimulates expression of cocaine and amphetamine regulated transcript (CART)
- Increased leptin levels in the blood stimulates weight loss to a certain extent. Moreover, leptin protects the body against weight loss in times of nutritional deprivation.
- Other factors demonstrated to influence long-term hunger and food intake regulating include insulin and circadian rhythm.
- Projections from cerebral loci, such as the limbic system and the cerebral cortex, on the hypothalamus also play a role in mediating appetite.
Why do we eat?
Delia Smith: "Food is for eating, and good food is to be enjoyed...I think food is, actually, very beautiful in itself."
Guy Fieri: "Food is not just eating energy. It's an experience".
- Known as consuming, eating is a process heterotrophic organisms perform to ingest food in order to gain energy and grow their bodies in order to survive. Different types of animals and heterotrophs eat different types of foods to ensure their own survival.
- For instance, carnivores eat animal matter, herbivores eat plant matter, omnivores eat a combination of both animal and plant matter, and detritivores eat detritus.
- Animals digest their food inside their bodies, whereas fungi digest organic matter outside their bodies.
What are the common eating practices amongst humans?
- Every home has a kitchen designated for cooking and preparation of meals and drinks. Those meals and drinks are subsequently served on the dinner table typically located in the dining room, dining hall or any area devoted for eating.
- If humans choose to eat outside of their home, they usually travel to restaurants, cafes, food courts, or food vendors/trucks because they don't have the time to prepare food, or they are attending a social occasion.
- Picnics, potlucks, and food festivals are other eating locations for the purpose of social gathering.
- Every day, humans typically have 2 or 3 meals a day (breakfast and lunch or brunch, dinner), along with snacks in between meals.
- Physicians recommend humans to consume 3 meals daily with each meal containing between 400 and 600 kilocalories, with a 4 to 6 hour gap between each meal.
- Nutritionists recommend 3 balanced meals containing 50% vegetables, 25% protein food such as meat, and 25% carbohydrates such as rice and pasta, which adds up to between 1800 and 200 kilocalories (the average required caloric count for a healthy person).
How did humans develop this ability to eat?
- The first thing newborn babies consume is either breast milk or milk formula.
- When the infant is around 2 months old, they are spoon-fed meagre amounts of pureed food since their teeth and immune systems haven't fully developed at this stage.
- A majority of infants will begin to eat adult foods when they are between 6 and 8 months old.
- Between 8 and 12 months of age, the digestive system further develops, which allows babies to begin eating finger foods. At this age range, a majority of babies lack molars or canines, and have a small number of incisors, which limits their diet.
- By 18 months, babies will have sufficiently developed teeth and an adequately mature digestive system to eat the same foods as adult humans.
- Every child generates mess as they're learning to eat various foods with a range of tastes, which can frustrate parents. Children generally don't understand the meaning of eating etiquette or neatness until they're around 5-6 years old.
- The most common eating position in the world is sitting on a chair in front of the table or bench. However, a common eating position in a majority of Middle Eastern cultures and some Asian cultures is sitting on the floor, which is thought to be healthier than eating while sitting in front of a table.
- The Ancient Greeks (for a symposium), the Ancient Romans, and the Ancient Hebrews (for Passover) preferred to eat in a reclining position.
How do other animals eat food?
i. Mammals
- Mammals require a nutritious and energy-rich diet in order to maintain a high body temperature. Mammals can be either carnivores (animal meats), herbivores (plants, seeds, leaves, fruits, nectar, gums, fungi), or omnivores.
- The digestive tract of an herbivore contains bacteria that ferment complex molecules in order to make them available for digestion, which are either situate in the stomach or in a large cecum.
- Some mammals, such as flies, dung beetles and termites, consume faeces in order to absorb the undigested nutrients when the food was first ingested. They are referred to as coprophagous.
- Carnivorous mammals generally have a regular digestive tract because not much specialised digestion processes are required to break down lipids, minerals and proteins found in meat. Sanders et al. (2015) noted the baleen whale as an exception because its gut is home to flora in a multi-chambered stomach, which function like terrestrial herbivores.
- According to Allen's rule, an animal's diet type is determined by its size. Since smaller mammals have a higher ratio of heat-releasing surface area to heat-generating volume, their energy requirements and metabolic rate tend to be greater.
- Mammals weighing less than 510 g (1.1 lb, 18 ounces) are mainly entomophagous (insect-eating) because they are unable to tolerate the slower, more complex digestive process of an herbivore.
- In contrast, Speaksman (1996) stated larger animals generate more heat and expel less heat, which means they can withstand either a slower collection process (canivores) or a slower digestive process (herbivores).
- Moreover, mammals weighing more than 510 g (1.1 lb, 18 ounces) typically can't gather an adequate amount of insects during their waking hours to sustain themselves.
- Wilson & Burnie (2001) found the only large entomophagous mammals tend to feed on large insect colonies such as ants or termites.
- Some mammals are omnivores that demonstrate varying levels of carnivore and herbivore diets, with a preference for one over the other due to the differences in which plants and meat are digested. For example, a majority of bears are herbivorous and some species may be carnivorous.
- Those mammals are categorised into mesocarnivore (50-70% meat), hypercarnivore (70% and greater of meat), and hypocarnivore (50% or less of meat). Hypocarnivores (e.g. American black bear) tend to have dull, triangular carnassial teeth to help them grind food, whereas hypercarnivores (e.g. polar bear) tend to have conical teeth and sharp carnassials to help them slash food, as well as strong jaws to crush bones.
- Some mammals are behaviourally described as omnivores, but they are physiologically categorised as either carnivores or herbivores. It is argued that animals had to gain both energy and nutrients from consuming plant and animal matter in order to be physiologically omnivorous.
- Singer & Barnays (2003) asserted that such omnivores are labelled as either carnivores or herbivores when they gain nutrients from sources that don't match their classification.
- Hutson, Burke & Haynes (2013) found some ungulates, such as camels, cattle, and giraffes, gnaw on bones in order to consume specific minerals and nutrients.
- Although cars are generally considered to be obligate carnivores, they occasionally consume grass in order to regurgitate inedible material (e.g. hairballs), augment haemoglobin production, and serve as a laxative.
- When food requirements can't be met, numerous mammals suppress their metabolism and conserve energy in a process known as hibernation. Humphries, Thomas & Kramer (2003) found that larger mammals, such as bears, become polyphagic in the period before hibernation in order to increase fat stores, whereas smaller mammals tend to collect and store food.
- A slower metabolism is accompanied by a reduced heart and respiratory rate, and reduced internal temperatures, which is roughly ambient temperature in certain cases.
- e.g. A hibernating arctic ground squirrel can decrease its internal temperature to about - 2.9°C (26.8 °F), however its head and neck region always never reduce below 0°C (32°F).
- Fritz (2010) noticed a few mammals, such as the fat-tailed dwarf lemur, in hot environments aestivate during periods of drought or severe heat.
ii. Birds
- Gill (1995) stated that birds consume a variety of foods such as carrion, fruit, nectar, seeds, plants, and the meat of small birds.
- Gionfriddo & Best (1995) explained the bird's digestive system includes a crop for storage and a gizzard for grinding food using swallowed stones in order to compensate for the lack of teeth. Hagey et al. (2010) found some bird species, such as pigeons, and some psittacine species lack a gallbladder.
- Sir David Attenborough (1998) described how a majority of birds are adapted for rapid digestion in order to augment flight. Battley et al. (2000) added how some migratory birds adapted to utilise the protein stored in different regions of their bodies, such as intestines, as supplemental energy during migration.
- Birds that devise numerous strategies to gain food or feed on a diverse range of food are labelled generalists, while other birds that target specific food items or employ a single strategy to gain food are labelled as specialists.
- For instance, a majority of birds glean for invertebrates, insects, fruit, or seeds, whereas some birds hunt insects by rapidly attacking from the tree branch.
- Reid (2006) described those bird species that prey on pest insects as 'biological control agents' in the biological pest control programmes.
- Nyffeler et al. (2018) estimated that entomophagous consumed between 400 and 500 million metric tons of arthropods annually.
- Paton & Collins (1989) found birds that feed on nectar such as hummingbirds, lorikeets, lories and sunbirds amongst others contain adapted brushy tongues and bills designed to fit co-adapted flowers.
- Baker & Baker (1973) noted that kiwis and shorebirds with long bills hunt for invertebrates, which may vary in length that result in the separation of ecological niches.
- Schreiber & Burger (2001) stated auks, diving ducks, loons and penguins use their feet or wings to propel themselves underwater to catch their prey. On the other hand, kingfishers, sulids and terns plunge dive from a tremendous height in the air to catch their prey.
- Flamingoes, certain species of prion and ducks are described as filter feeders, and geese and dabbling ducks are described as grazers.
- Some birds, such as frigatebirds, gulls, and skuas steal food from other birds, a behaviours known as kleptoparasitism. Vickery (1994) estimated great frigatebirds stole at most 40% and on average 5% of food from masked boobies.
- Hiraldo et al. (1991) found other birds, such as vultures, are scavengers that eat carrion, whereas other birds such as gulls and corvids are opportunities.
What is biting?
- When animals actively and rapidly close their jaw around an object, they are described as biting, a common zoological behaviour. Animals with teeth such as amphibians, fish, mammals, reptiles, as well as some arthropods, are able to engage in biting.
- Biting occurs as a result of myocytic contraction of the muscles of mastication, which generates force that triggers preparatory jaw abduction (opening), followed by rapid jaw adduction (closing) by shifting the top and bottom teeth towards each other.
- Macro-organisms that can bite are able to build, eat, forage, fight, play and protect, etc. Some animals bite as a demonstration of physical aggression against other animals because of predatory or territorial intentions.
- Other animals bite for the purposes of eating, carrying objects, softening and preparing food for its offspring, removing ectoparasites or irritating foreign objects from its body surface, scratching itself, and grooming other animals.
- Animal bites can lead to avulsions, fractures, envenomation, haemorrhages, infections, punctures, or worse, death. For example, dog bites are quite common in modern human societies, with children being the most common victim.
- Other animals that can attack humans include feral cats, spiders, snakes, micropredators such as vampire bats, bedbugs, lice, fleas, mosquitoes and ticks, and wild carnivores such as big cats, bears, crocodiles, barracudas, piranhas and sharks, wolves.
What types of teeth are used for biting?
- A 2018 study found carnivores have canine, carnassial, and molar teeth, while herbivores have incisor teeth and wide-back molars. It is suggested carnivores use their long and sharp teeth to both grip their prey and rip their prey into chunks. However, they don't have flatter teeth to help them chew the food into smaller particles, thus they have to swallow food in large chunks.
- e.g. Great white sharks contain broad, serrated teeth that allow them to prey on large marine animals.
- In contrast, herbivores contain rows of wide, flat teeth to bite and chew grass, as well as other plants. e.g. Cows spend up to 11 hours a day biting off grass and using its wider and flatter teeth to grind it with their molars.
- Omnivores, such as humans, contain both flat teeth and sharp teeth, therefore they are able to consume both meat and plants.
How do animals bite to carry things?
- Some animals, such as beavers and ants, bite large objects as part of a carrying mechanism due to the sheer amount of force exerted by their teeth.
- For example, Müller-Schwarze (2011) found beavers have large front teeth that allow them to gnaw large chunks of wood, as well as strong jaw muscles to bring down tall trees and carry them back to their dam.
- Nguyen, Lilly & Castro (2014) found ants have strong jaws to carry objects significantly heavier than their body mass to forage for their colonies.
- Drees (2002) found fire ants have strong jaws to bite on their prey, and subsequently inject a toxin via their stinger to subdue them and carry their prey back to their territory.
How dangerous are animal bites?
- A majority of snakes bite other organisms to inject their venomous saliva, which contain at least one of the major types of toxins, including cytotoxins, hemotoxins, myotoxins, and neurotoxins.
- Spider venom contains polypeptides that bind to certain ion channels, which excites certain parts of the central, peripheral and autonomic nervous systems. This results in hyperactive release of neurotransmitters and subsequent refractory paralysis.
- Spiders bite other organisms as a form of predation, as well as a form of self-defence or an attempt to escape.
- Braitberg & Segal (2009) found venom from the bites of recluse spiders and widow spiders contain neurotoxins and necrotising agents that paralyse and consume prey.
- Mosquito bites are considered non-lethal, but they can trigger allergic wheals that become itchy and lasts a few days. In some regions, they can spread blood-borne diseases, such as malaria and West Nile fever, by transmitting protozoic or viral pathogens.
- In 2019, the Centre for Disease Control described a number of diseases being spread through tick bites endemic to their location, such as Lyme disease, African tick bite fever, Colorado tick fever, tick-borne encephalitis, etc.
Describe biting in humans
- Human children aged 30 months and younger that learn to bite is regarded an age-appropriate behaviour and reaction.
- In contrast, children older than 30 months are expected to gain verbal skills to communicate their desires and dislikes, since biting isn't regarded as an age-appropriate behaviour.
- Children can be taught to avoid biting by a range of methods such as redirection, or changing the environment and responding to biting by discussing appropriate ways to express anger and frustration.
- A 2011 report found school-age children older than 30 months that habitually bite are recommended to receive professional intervention.
- Robsam et al. (2016) stated the physical fights or acts of self-defence may be initiated due to a bite.
What is chewing?
- Also known as mastication, chewing is a process that involves food being crushed and grounded by the teeth. It is considered to be the first step of digestion, as chewing increases the surface area of the food in order for enzymes to break them down more efficiently.
- During the chewing process, the tongue and cheek places the food between the teeth where it gets ground down. The muscles of mastication move the jaws together in order for the teeth to intermittently contact each other, then repeatedly occlude and open.
- Chewing softens and warms the food inside the mouth because the enzmes in saliva help break down carbohydrates in the food.
- After a period of chewing, the chewed food becomes a bolus, which gets pushed down by the tongue past the uvula to be swallowed. The food subsequently travels through the oesophagus downwards via peristalsis to the stomach, where the next phase of digestion occurs.
- Miquel-Kergoat et al. (2015) found a directly proportional relationship between the level of chewing and the levels of relevant gut hormones. Moreover, increasing the level of chewing is known to decrease self-reported hunger and food intake.
Describe the chewing motor program
- Chewing is mainly an semi-autonomic behaviour, which can be influenced by higher conscious input. The motor program for chewing is thought to be controlled by the central nervous system.
- Feedback from proprioceptive nerves situated in the teeth and the temporomandibular joints determine how the neural pathways are generated, which in turn influence the duration and force of individual activation of muscle fibre groups in the masseter and temporalis muscles.
- Peyron et al. (2004) thought that this motor program continuously adapt to changes in the type of food being ocnsumed or occlusion, which is regarded as a learned skill.
What muscles are involved in chewing?
There are 4 classical muscles of mastication, which are:
- Masseter (consists of the superficial and deep head)
- Temporalis (debate whether the sphenomandibularis is part of this muscle or not)
- Medial pterygoid
- Lateral pterygoid
The secondary or accessory muscles are:
- Buccinator
- Infrahyoid muscles (the sternohyoid, sternothyroid, thyrohyoid, and omohyoid muscle)
- Suprahyoid muscles (digastric muscle, mylohyoid muscle, and geniohyoid muscle)
- The mandible, or lower jaw, is attached to the temporal bone of the skull via the temporomandibular joint, which is a complex joint that allows movement in all planes. The muscles of mastication arise from the skull and insert into the mandible, which permits jaw movements during contraction.
- Each of the primary muscles of mastication comes in pairs, with each side of the mandible containing 1 of the 4 muscles.
- The muscles of mastication are innervated by the trigeminal nerve (CN V), or specifically, the mandivular branch (V3).
- The muscles of mastication embryologically originate from the first pharyngeal arch, whereas the muscles of facial expression originate from the second pharyngeal arch.
- Chewing stimulates the production of saliva and triggers the sensory perception of the food being consumed, which regulates the timing of the food being swallowed.
- Cassady et al. (2009) suggested that chewing almonds between 25 and 40 times increases satiety, as well as increases the nutrients being extracted from the almonds. Miquel-Kergoat et al. (2015) found chewing reduces self-reported hunger and thus food intake.
- Foods that don't require chewing either by choice or for medical reasons such as tooth loss, is regarded as a soft diet. N'Gom & Woda (2002) thought soft diets may result in malnutrition because of the reduced intake of fruit and vegetables.
- Smith, Miquel-Kergoat & Thuret found chewing also stimulates the hippocampus, as well as neurogenesis in the hippocampus in both humans and mice.
What is swallowing?
- Also referred to as deglutition, swallowing is the process of allowing food, drink or medicine to travel from the mouth, to the pharynx, and into the oesophagus, while closing the epiglottis.
- If an animal or human is unable to fully swallow the food, drink, or medicine, and instead travels through the trachae, then they will experience choking or pulmonary aspiration.
- In the human body, the swallowing reflex controls the automatic temporary closing of the epiglottis.
How do humans swallow?
- There are 3 phases in the eating and swallowing processes: oral, pharyngeal, and oesophageal phases, with each phase being controlled by a unique neurological mechanism.
i. Oral Phase
The oral phase occurs voluntarily, which means it's controlled by the medial temporal lobes and the limbic system of the cerebral cortex, as well as the motor cortex and other cortical areas.
- The mandible depresses and the lips abduct to allow food or liquid to enter the oral cavity.
- Upon entering the oral cavity, the mandible lifts and the lips adduct in order to contain the food and liquid inside the oral cavity.
- Saliva released by the salivary glands (stimulated by parasympathetic nerves) moistens the food.
- Food is then mechanically disintegrated by the teeth controlled by the muscles of mastication (V3) that stimulate the temporomandibular joint.
- The tongue rolls the bolus from one side of the oral cavity to the other.
- Buccinator (VII) muscle contains the food against the teeth's occlusal surfaces.
- When the bolus is stabilised by saliva, perceived by the lingual nerve of the tongue (VII - chorda tympani, and IX - lesser petrosal) (V3), it can be swallowed. If the food is too dry, then the bolus can't be swallowed.
- The intrinsic muscles (XII) creates a trough at the back of the tongue.
- The trough strikes against the hard palate from front to back, which pushes the bolus to the back of the tongue.
- The tongue then lifts to the roof of the mouth (stimulated by the mylohyoid [mylohyoid nerve—V3], genioglossus, styloglossus and hyoglossus [the rest of XII]) such that the tongue slopes downwards posteriorly.
- The genioglossus and styloglossus (both XII) muscles contract to help form the the central trough.
- After the oral preparatory phase, the food bolus is created and is on the point of being directed posteriorly into the pharnyx.
- The orbicularis oris contracts and adducts the lips to create a tight seal of the oral cavity.
- The superior longitudinal muscle lifts the apex of the tongue to touch the hard palate and the bolus is pushed to the posterior area of the oral cavity.
- When the bolus approaches the palatoglossal arch of the oropharynx, this initiates the pharyngeal phase of swallowing.
- There are receptors situated over many locations: the base of the tongue, the tonsillar fossa, the palatoglossal and palatopharyngeal arches, uvula and posterior pharyngeal wall.
- Those receptors trigger the swallowing reflex, which are proprioceptive. The afferent limb of the reflex is CN IX nerve, and the efferent limb is the pharyngeal plexus [CN IX and X].
- Stimuli from these receptors then trigger the pharyngeal phase. The swallowing reflex can be triggered entirely by peripheral stimulation of the internal branch of the superior laryngeal nerve.
ii. Pharyngeal Phase
- The pharyngeal phase is initiated by the oral phase, which is subsequently coordinated by the swallowing centre on the medulla oblongata and pons.
- The swallowing reflex is triggered by touch receptors located in the pharnyx as the tongue pushes a bolus of food to the back of the mouth, or by stimulation of the palate (palatal reflex).
- This phase is voluntary and involves the following cranial nerves: CN V (trigeminal), VII (facial), and XII (hypoglossal).
- The pharyngeal phase requires all other exits from the pharynx to be occluded, including the nasopharynx and the larnyx. When the pharyngeal phase is initiated, other activities such as breathing, chewing, coughing and vomiting are consequently inhibited.
- The tensor palatini (Vc) increases the tension of the soft palate, which is subsequently raised by the levator palatini (innervated by pharyngeal plexus [CN IX, X]) to close the nasopharynx.
- The walls of the pharynx simultaneously move closer to the posterior free border of the soft palate, which is stimulated by the palatopharyngeus (innervated by pharyngeal plexus - [CN IX, X]) and the upper part of the superior constrictor (innervated by pharyngeal plexus - CN IX, X]).
- The suprahyoid and longitudinal pharyngeal muscles - stylopharyngeus (CN IX), salpingopharyngeus (innervated by the pharyngeal plexus [CN IX, X]) and palatopharyngeus (innervated by the pharyngeal plexus - [CN IX, X]) pulls the pharynx upwards and forwards in order for the bolus to flow through.
- The superior constrictor muscles bring the palatopharyngeal folds on each of the pharynx closer together in order to allow a small bolus through.
- The levator palatini (innervated by pharyngeal plexus - [CN IX, X]), tensor palatini (Vc) and salpingopharyngeus (innervated by pharyngeal plexus - [CN IX, X]) act to close the nasopharynx and lift the pharynx to open the auditory tube.
- This equalises the pressure between the nasopharynx and the middle air. Although it doesn't play a role in swallowing, it occurs as a result of it.
- The palatoglossus (innervated by pharyngeal plexus - [CN IX, X]) and the intrinsic muscles of the tongue (CN XII) and styloglossus (CN XII) keeps the oropharynx closed.
- To prevent aspiration during swallowing, the true vocal folds close as part of a laryngopharyngeal protective mechanism.
- Contraction of the lateral cricoarytenoids and the oblique and transverse arytenoids (all recurrent laryngeal nerve of vagus) result in the adduction of the vocal cords. Since the true vocal folds adduct during swallowing, a finite period of apnoea has to occur with each swallow.
- Swallowing is observed to usually occur during expiration, possibly to clear the upper larynx from food remnants or liquid. This finding is clinical significant because patients with compromised lung function tend to develop respiratory distress as a meal progresses.
- Subsequently, the false vocal folds adducts, the aryepiglottic folds adducts and the epiglottis turns backwards.
- The aryepiglotticus (recurrent laryngeal nerve of vagus) contracts, which results in apposition of the arytenoids (i.e. closes the laryngeal aditus by coalescing the aryepiglottic folds), and pulls the epiglottis downwards in order for its lower half to contact the arytenoids, thus closing the aditus.
- Retroversion of the epiglottis helps to anatomically direct the food bolus laterally towards to the piriform fossa.
- Moreover, the larynx is elevated along with the pharynx under the tongue by the stylopharyngeus (CN IX), salpingopharyngeus (pharyngeal plexus - [CN IX, X]), palatopharyngeus (pharyngeal plexus - [CN IX, X]) and inferior constrictor (pharyngeal plexus - CN [IX, X]).
- The pharyngeal phase is passively and reflexively regulated by CN V, X (vagus), XI (accessory) and XII (hypoglossal).
- The respiratory centre of the medulla is inhibited by the swallowing centre for a brief moment during which swallowing occurs. This indicates that breathing is impossible during the pharyngeal phase of swallowing and the moment where breathing is inhibited is called deglutition apnoea.
- The hyoid is lifted by the digastric (CN V & CN VII) and stylohyoid (CN VII), which elevates the pharynx and larynx even further.
- The bolus travels down towards the oesophagus by pharyngeal peristalsis, which occurs by sequential contraction of the superior, middle and inferior pharyngeal constrictor muscles (pharyngeal plexus - CN IX, X).
- The lower section of the inferior constrictor (cricopharyngeus muscle) is usually closed and only opens when the bolus approaches it. Contrary to popular belief, gravity actually plays a minor role in the swallowing process because it is possible to swallow a food bolus when upside down.
- The velocity of the bolus through the pharynx depends on several factors such as viscosity and volume of the bolus. Clave et al. (2006) calculated the bolus velocity in healthy adults is roughly 30 - 40 cm/s.
iii. Oesophageal Phase
This phase of swallowing occurs due to involuntary neuromuscular control. However, the speed of the food bolus in the oesphagus is significantly slower than in the pharynx.
- The bolus enters the oesophagus and is driven downwards initially by striated muscle (recurrent laryngeal [CN X]), followed by the smooth muscle (CN X) at a rate of 3 - 5 cm/s.
- The upper oesphageal sphincter relaxes to allow the passage of the food bolus.
- Then the food bolus is sequentially propelled through the oesophagus into the stomach by the striated constrictor muscles of the pharynx as well as peristalsis and relaxation of the lower oesophageal sphincter.
- The larynx and pharynx subsequently shift down from the hyoid to their relaxed positions by elastic recoil.
- Some people learn to swallow fluidly without closing their mouth by solely controlling their tongue and jaw to propel fluids or food down their oesophagus. This is known as M-type swallowing.
- With a continuous motion, a person can forge breathing and prioritise the matter being swallowed. Sword swallowers are known to employ this technique of swallowing.
How do non-mammals swallow?
- A majority of birds contain oesophagus that is nothing more than a gravity chute, e.g. a seagull swallowing a fish or a stork swallowing a frog. Birds typically lift their head with its beak pointing up and forcing its prey inside and downwards with its tongue and jaws.
- Fish pump water into its mouth and out of its gills in order to direct the food to the back of the pharynx because its tongue is mainly bony and limited in mobility.
- Snakes swallow their prey by raking with their lower jaw until the prey is adequately deep inside to be propagated by body undulations.
What is digestion?
- Digestion is the process of large insoluble food substances disintegrating into smaller water-soluble molecules in order to become absorbed into the blood plasma. In some organisms, these smaller food molecules are absorbed through the small intestine into the blood stream.
- Digestion is a form of catabolism that is classified into 2 processes depending on the method of breaking down food: chemical and mechanical digestion.
- Mechanical digestion refers to the physical breakdown of large chunks of food into smaller pieces, which become accessible to digestive enzymes. This process occurs in the mouth through mastication and in the small intestine through segmentation contractions.
- Chemical digestion refers to the enzymatic breakdown of large food substances into smaller compounds to be utilised by the body.
What is the digestive system?
- There are different types of digestive systems, but they are mainly categorised into internal and external digestive systems.
- David Dusenbery (1996) thought external digestion developed early in evolutionary history, since a majority of fungi depend on it. External digestion involves the secretion of enzymes into the environment surrounding the organism, where they disintegrate the organic material, with some of the products diffuse back to the organism.
- In contrast, internal digestion occurs inside an animal's tube i.e. gastrointestinal tract. Dusenbery (2006) believed this process is more efficient since a large proportion of the disintegrated products can be absorbed, and the internal chemical environment can be efficiently regulated.
- Some organisms, such as spiders, secrete biotoxins and digestive chemicals (e.g. enzymes) into the extracellular environment before they ingest the "soup" of its prey. Once the food or nutrients has entered the organism, digestion occurs in a vesicle or a sac-like structure, through a tube, or through a number of specialised organs with the purpose of maximising the efficiency of nutrient absorption.
What are the different types of secretion systems?
i. Channel transport system
- Channel transport systems involve a number of proteins create a contiguous channel that traverse the inner and outer membranes of the bacteria. It consists of 3 main protein subunits: the ATP-binding cassette transporter, membrane fusion protein (MFP), and outer membrane channel protein.
- This system transport various types of chemicals, such as ions, pharmacological drugs, to proteins of various sizes (20 - 900 kDa).
- Wooldridge (2009) found those chemical species vary in size from the Escherichia coli peptide colicin V (10 kDa) to the Pseudomonas fluorescens cell adhesion protein LapA of 900 kDa.
ii. Molecular syringe
- A molecular syringe is a type III secretion system used mainly by bacteria (e.g. certain types of Salmonella, Shigella, Yersinia) to inject nutrients into protist cells.
- Salyers & Whitt (2009) found this secretion system in Yersinia pestis and demonstrated that toxins were injected directly from the bacterial cytoplasm into the cytoplasm of host cells rather than secreted into the extracellular medium.
iii. Conjugation machinery
- The conjugation machinery allows certain bacteria (and archael flagella to transport both DNA and proteins. Cascales & Christie (2003) discovered this system in Agrobacterium tumefaciens, which introduces the Ti plasmid and proteins into the host in order to form the crown gall (tumour). In addition, Christie et al. (2005) found the VirB complex of Agrobacterium tumefaciensis the prototypic system.
- Conjugation machinery is also found in the nitrogen-fixing Rhizobia, which includes the Agrobacterium Ti or Ri plasmid that gets transferred to plant cells.
- When transferred genes enter the plant cell nucleus, they effectively transform the plant cells into opine factories, which the bacteria use as sources of carbon and energy. The infected plant cells would produce crown gall or root tumours.
- Therefore, the Ti and Ri plasmids are the endosymbionts of the bacteria, which are in turn endosymbionts of the infected plant.
- The Ti and Ri plasmids are conjugative because they transfer between bacteria via an independent system (the transfer (tra) operon) from that for inter-kingdom transfer (the virulence (vir) operon). This results in the creation of virulent strains from previously avirulent Agrobacteria.
iv. Release of outer membrane vesicles
- Gram-negative bacteria pinch off a small portion of its outer membrane to create a spherical structure composed of lipid bilayer that contain the periplasmic materials called an outer membrane vesicle.
- Vesicles from a number of bacterial species were discovered to contain virulence factors with variable effects, such as immunomodulation, intoxication of host cells.
- McBroom & Kuehn (2007) found that the process of loading cargo proteins into vesicles was selection, even in response to stress conditions.
What is the gastrovascular cavity?
- The gastrovascular cavity is the primary organ of digestion and circulation in 2 major animal phyla: the Coelenterates or cnidarians (including jellyfish and corals) and Platyhelminthes (flatworms).
- The gastrovascular system in cnidarians is called the coelenteron, also known as a "blind gut" or "blind sac", because food enters and waste exits through the same orifice.
- The radially symmetrical cnidarians contain a sac-like body in 2 distinct layers, the epidermis and gastrodermis, with a jelly-like layer called the mesoglea.
- Extracellular digestion typically occurs within the central cavity of the sac-like body, which contains only 1 orifice to the external environment. In a majority of cnidarians, this cavity is surrounded by tentacles for catching prey.
What is a phagosome?
- A phagosome is a vacuole that forms around a particle absorbed by phagocytosis, which is formed by the fusion of cell membrane. It is a cellular compartment in which pathogenic microorganisms are digested.
- They fuse with lysosomes during the maturation stage to form phagolysosomes. I'll delve more into this process in another post.
Overview of vertebrate digestion
In a majority of vertebrates, digestion is divided into a number of main processes:
b. Breakdown of food = Food is disintegrated both mechanically and chemically. This involves mastication and the blending of the resulting bolus with acids, bile, water and enzymes in the stomach and intestine to break down complex chemical compounds into simple chemical structures.
c. Absorption = Nutrients from the digestive system enter the circulatory and lymphatic capillaries via osmosis, active transport, and diffusion.
d. Excretion (Egestion) = Elimination of undigested food contents from the digestive tract through defecation.
I will detail the anatomical structures, and biochemical and physiological mechanisms occurring in each organ of the digestive system.
Describe the human digestion process
The human digestive involves the gastrointestinal tract as well as the accessory organs of digestion such as the gallbladder, liver, pancreas, tongue and salivary glands. The process of digestion has 3 stages: cephalic phase, gastric phase, and intestinal phase
What are the stages of the human digestion?
A. Cephalic phase
This stage is initiated by the secretions of chemicals from the gastric glands in response to the human seeing and smelling food. It includes the following processes: the mechanical breakdown of food by biting and chewing, the chemical breakdown of food by digestive enzymes (in the saliva), which occurs in the mouth. Saliva is secreted by the salivary and serous glands on the tongue, which contains the digestive enzymes. Chewing helps mix the food with saliva to produce a bolus that can be swallowed down the oesophagus to the enter the stomach.
1. Mouth
- The mouth is the first portion of the alimentary canal that receives food and produces saliva. The mucous membrane epithelium lining the interior of the mouth is called the oral mucosa.
Describe the development of the mouth
- A vertical depression forms between the philtral ridges between the upper lip and the nasal septum called the philtrum. It is located where the nasomedial and maxillary processes connect during the development of the embryo. If these processes fail to fuse correctly, this leads to a cleft lip and/or a cleft palate.
- The deep creases of tissue that extend from the nose to the sides of the mouth are called the nasolabial folds. The increase in the prominence of the nasolabial folds is one of the first signs of age on the human face.
Describe the structure of the mouth
i. Oral Cavity
- The mouth consists of 2 regions: the vestibule and the oral cavity proper. The vestibule is located between the teeth, lips and the cheeks. The oral cavity is bounded at the sides and by the alveolar process in front and by the isthmus of the fauces at the back.
- The roof of the mouth is formed by the hard palate at the front, and the soft palate at the back.
- The uvula protrudes downwards from the middle of the soft palate at the back of the oral cavity.
- The floor of the mouth is formed by the mylohyoid muscles and primarily occupied by the tongue.
- The oral mucosa lines the sides and the under surface of the tongue to the gums, as well as the inner part of the mandible (jaw). This region receives secretions from the submandibular and sublingual salivary glands.
ii. Lips
- The lips are a vertical pair of soft appendages joined to the jaws and are the most visible section of the mouth of numerous animals, including humans.
- Vertebrate lips are soft, movable and contribute to the facilitation of food ingestion (by gulping and suckling) and the articulation of sound and speech.
- The lips combine to close the opening of the mouth, which form a line between the upper and lower lip.
Describe the structure of the lips
- The upper and lower lips are known as the "Labium superius oris" and "Labium inferius oris", respectively.
- The juncture where the lips link up with the surrounding skin of the mouth region is called the 'vermillion border', and the reddish area within the borders is called the 'vermillion zone'.
- The vermillion border of the upper lip is called the 'cupid's bow'.
- The fleshy protuberance in the centre of the upper lip is known as a 'tubercle', sometimes called the procheilon (prochilon), the "tuberculum labii superioris", and the "labial tubercle".
- The vertical groove that runs from the procheilon to the nasal septum is known as the philtrum.
- The skin of the lip typically has 3 - 5 cellular layers, which is thinner than typical face skin (16 layers). The lips' skin form the border the between the exterior skin of the face, and the interior mucous membrane of the mouth's interior.
- Lips with a lighter skin colour indicates low melanocyte levels on the skin, which makes the blood vessels appear through the skin of the lips, leading to the typical red colouring.
- Lips with darker skin colour have higher melanocyte levels, which means it contains more melanin on the skin.
- Since lip skin doesn't have hair follicles and sweat glands, they don't have the usual protective layer of sweat and body oils to maintain the skin's smoothness, inhibit pathogens, and regulate heat. Therefore, lips can dry out quicker than body skin and become chapped more easily.
- The lower lip is formed by a branch of the first pharyngeal arch called the mandibular prominence, and it covers the anterior body of the mandible. The depressor labii inferioris muscle moves the lower lip downwards and the orbicularis oris muscle borders the lower lip inferiorly.
- The upper lip covers the anterior part of the body of the maxilla. Its upper half shares same colour as of typical skin colour and has a depressed centre, which is directly beneath the nasal septum, called the philtrum (Latin for "lower nose").
- The upper lip's lower half has a different, red-coloured skin tone that is a similar shade to the red-coloured surface of the mouth's interior .Therefore, the term vermillion is used to refer to the coloured portion of either lip. The levator labii superioris lifts the upper lip, joined to the lower lip by the thin lining of the lit itself.
- 2 common facial features of foetal alcohol syndrome are a flat philtrum and thin vermillion of the upper lip.
i. Microanatomy
- The skin of the lips is composed of stratified squamous epithelium.
- The mucous membrane is perceived by a section in the sensory cortex, hence its high sensitivity.
- The frenulum of the lower lip is called the frenulum labii inferioris, whereas the frenulum of the upper lip is called the frenulum labii superioris.
- The lips are primarily innervated by the trigeminal nerve, which is divided into the maxillary branch and mandibular branch.
- The upper lip, as well as the skin of the face between the upper lip and the lower eyelid (except for the bridge of the nose) is innervated by the infraorbital nerve, which is branch of the maxillary branch.
- The skin and the mucous membrane of the lower lip and the (anterior) labial gingiva are innervated by the mental nerve, which is a branch of the mandibular branch (via the inferior alveolar nerve).
iii. Blood supply
- Both upper and lower lips are supplied by the superior and inferior labial branches of the facial artery (respectively), which is 1 of the 6 non-terminal branches of the external carotid artery.
- Each of the 2 branches bifurcate and anastomose with their accessory branch from the other terminal.
iv. Muscles
The muscles responsible for lip movement include:
- Buccinator
- Orbicularis oris
- Modiolus = Anchor point for several muscles
- Muscles that elevate the lips = Levator labii superioris, levator labii superioris alaeque nasi, levator anguli oris, zygomaticus minor, zygomaticus major
- Muscles that depress the lips = Risorius, depressor anguli oris, depressor labii inferioris, mentalis
- All muscles of facial expression are derived from the mesoderm of the 2nd pharyngeal arch and thus are supplied by the nerve of the 2nd pharyngeal arch called the facial nerve (CN VII).
- They are all specialised members of the panniculus carnosus, which connect to the dermis and dimple or wrinkle the overlying skin.
What are the functions of the lips?
ii. Food intake
- Lips can serve to hold food or move it to the mouth using its own muscles and adjacent muscles. Furthermore, they shut the mouth airtight in order to hold food and drink inside, and to prevent any unwanted objects out.
- The lips make a narrow funnel in order to increase the mouth's suction, which helps babies breast feed.
- The lips can change shape to suck in certain contexts, such as sucking on a straw to drink liquids.
ii. Articulation
- The lips can produce different sounds, such as labial, bilabial, and labiodental consonant sounds, as well as vowel rounding, which make it an essential component speech apparatus.
- The lips play important roles in whistling and the blowing of wind instruments such as the clarinet, flute, trumpet, and saxophone.
- People with hearing loss may consciously or unconsciously lip read in order to understand speech without requiring the perception of the actual sounds. They use visual cues from the lips to perceive what sounds may have been heard, e.g. the McGurk Effect.
iii. Tactile organ
- The lip contains numerous nerve endings, making it an important tactile sensory organ. They are sensitive touch and temperature, such as coldness and warmth. Therefore, the lips are an important aid to perceive and understand unknown objects for babies and toddlers.
iv. Erogenous zone
- Due to the abundance of nerve endings, the lips are an erogenous zone. Thus, they play an essential role in kissing and other intimate behaviours.
- Law Smith et al. (2011) found a woman's facial and sexual attractiveness is strongly associated with the composition of her hormones during puberty and development.
- It is known that the high oestrogen levels in women are directly associated with the maintenance of a relatively youthful facial structure during puberty and final maturation. In addition, high oestrogen levels are directly correlated with larger eyes and fuller lips, which are perceived as more feminine.
- Males are sexually attracted to a woman's lips because they are a biological indicator of a woman's health and fertility.
- An article from The London Times theorised that a woman apply lipstick or collagen lip enhancement to create the illusion that a woman has higher oestrogen than she actually has, and therefore appears to others as more fertile and attractive.
- It is thought that women are more attracted to men with masculine lips that are middle-sized (i.e. not too big or too small), and have a rugged and sensual appearance.
- A 2003 article stated that researchers discovered the most sexually attractive features in both men and women are a big eyes, a small nose and voluptuous lips.
v. Facial expression
- In facial expression, the mouth line is shaped like an up-open parabola in a smile, and like a down-open parabola in a frown.
- A down-turned mouth indicates a mouth line shaped like a down-turned parabola. Furthermore, a down-turned mouth is a sign of Prader-Willi syndrome.
iii. Nerve supply
- The teeth and the periodontium (tissues supporting the teeth) are innervated by the maxillary and mandibular nerves, which are divisions of the trigeminal nerve.
- The maxillary (upper) teeth and the connected periodontal ligament are innervated by the superior alveolar nerves, branches of the maxillary division, which include the anterior superior alveolar nerve, posterior superior alveolar nerve, and the middle superior alveolar nerve. These nerves combine to form the superior dental plexus located above the maxillary teeth.
- The mandibular (lower) teeth and the connected periodontal ligament are innervated by the inferior alveolar nerve, which is a branch of the mandibular division.
- This nerve extend inside the mandible, within the inferior alveolar canal beneath the mandibular teeth, which leads to branches to all of the lower teeth (inferior dental plexus).
- The oral mucosa of the gingiva (gums) located on the facial (labial) part of the maxillary incisors, canines and premolar teeth is innervated by the superior labial branches of the infraorbital nerve.
- The posterior superior alveolar nerve innervates the gingiva on the facial part of the maxillary molar teeth. The gingiva on the palatal part of the maxillary teeth is innervated by the greater palatine nerve except for in the incisor area, where in fact the nasopalatine nerve (long sphenopalatine nerve) innervates that area.
- The gingiva of the lingual part of the mandibular teeth is innervated by the sublingual nerve, which is a branch of the lingual nerve.
- The gingiva of the facial part of the mandibular incisors and canines is innervated by the mental nerve, which is an extension of the inferior alveolar nerve that appear from the mental foramen.
- The gingiva of the buccal (cheek) part of the mandibular molar teeth is innervated by the buccal nerve.
Describe the functions of the mouth
- The main functions of the mouth include eating, drinking, speaking and breathing (as temporary backup if the nose is obstructed).
- Infants use their mouth, lips and jaw to instinctively suck for nutrients as part of a sucking reflex.
- Some disabled people use their mouths to perform usual tasks that they normally use their hands for, such as illustrating (drawing or painting), typing, texting, and writing.
- On average, an adult male mouth can hold about 71.2 ml (2.51 imp fl oz; 2.41 US fl oz), whereas an adult female mouth can hold about 55.4 ml (1.95 imp fl oz; 1.87 US fl oz).
2. Salivary Glands
- In numerous vertebrates including mammals, the salivary glands are exocrine glands that produce saliva through a system of ducts.
- Humans have 3 pairs of major salivary glands called the parotid, submandibular, and sublingual glands, as well as 100s of minor glands. They are classified as mucous, serous, or seromucous.
a. Parotid glands
- The word parotid comes from the Greek παρωτίς (stem παρωτιδ-), which means (gland) behind the ear < παρά - pará : in front, and οὖς - ous (stem ὠτ-, ōt-) : ear. Therefore, it literally means "beside the ear".
Describe the structure of parotid glands
- The parotid glands are a pair of serous salivary glands situated below and in front of each ear canal, where they drain their secretions into the vestibule of the mouth via the parotid duct.
- Each gland is located behind the mandibular ramus and ahead of the mastoid process of the temporal bone.
- Fehrenbach & Herring (2012) stated that a human can feel the gland on each side of their face by touching the area in front of each ear, along the cheek, and under the angle of the mandible.
- The parotid duct is a long excretory duct that extends anteriorly from each gland, superficial to the masseter muscle. The duct penetrates the buccinator muscle, then connects to the mouth on the inner surface of the cheek, typically opposite the maxillary 2nd molar.
- The parotid papilla is a bit of elevated tissue that demarcates the opening of the parotid duct on the inner surface of the cheek.
- The parotid contains 4 surfaces - anteromedial, posteromedial, superior, superficial and lateral, 3 borders - anterior, medial, and posterior, and 2 ends - superior and inferior (apex).
From lateral to medial, the following structures pass through the gland:
- Branches of the great auricular nerve
- External carotid artery
- Superficial temporal artery
- Facial nerve
- Maxillary artery
- Retromandibular vein
- Sometimes accessory parotid glands as an anatomic variation, which are located close to the main glands and are composed of ectopic salivary gland tissue.
- The capsule of the parotid gland is formed by the investing layer of the deep cervical fascia, which is supplied by great auricular nerve.
- The splitting of the fascia occurs between the angle of the mandible and the mastoid process, which encloses the gland.
- The superficial lamina (parotidomassetric fascia) is thick and is connected to the zygomatic arch, whereas the deep lamina is thin and is connected to styloid process, tympanic plate and the ramus of the mandible.
- A section of deep lamina situated between the styloid process and the mandible is thickened to create the stylomastoid ligament.
Describe the histology of the parotid gland
- The parotid gland has a capsule composed of dense connective tissue that is also supplied a false capsule by the investing layer of the deep cervical fascia.
- The fascia is situated at the imaginery line between the angle of the mandible and the mastoid process. Embedded within the capsule is a small muscle called the risorius.
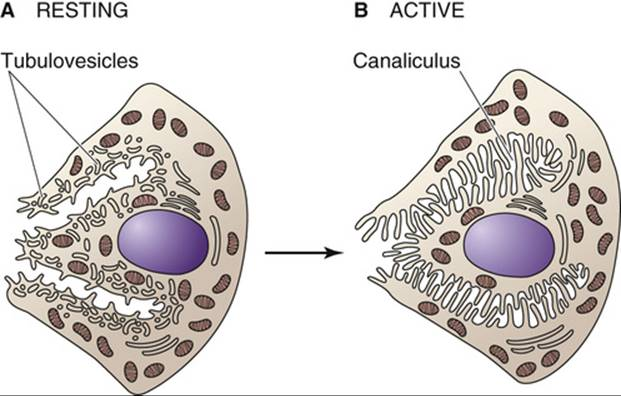
- Bath-Balogh & Fehrenbach (2010) found the gland contains both short, striated ducts and long, intercalated ducts. The intercalated ducts are lined with cuboidal epithelial cells and contain lumina that are bigger than those of the acini. On the other hand, the striated ducts are composed of simple columnar epithelium, with striations that represent the infolded basal cell membranes and mitochondria.
- Although the parotid gland is the largest salivary gland, it provides only a quarter of the total salivary volume. Bath-Balogh & Fehrenbach (2011) found the serous cell predominates in the parotid gland, meaning it releases a serous secretory product.
- The parotid gland secretes salivary alpha-amyalase (sAA), the first step in the breakdown of starches during mastication. I will explain the decomposition of starches later in the post.
Describe the development of the parotid gland
- The parotid salivary glands begin to form during the 6th week of the prenatal development, and are the first major salivary glands to appear.
- The epithelial buds of these glands are on the inner part of the cheek, close to the labial commissures of the primitive mouth, which runs from the ectodermal lining close to the angles of the stomodeum in the 1st / 2nd pharyngeal arches. Moore (2003) found the stomodeum is formed from the burst of the oropharyngeal membrane at about 26 days.
- These buds develop posteriorly toward the otic placodes of the ears and then deviate to form solid cords with rounded terminal ends close to the developing facial nerve.
- At the 10th week of prenatal development, these cords canalise to form ducts, with the largest one becoming the parotid duct for the parotid gland. The rounded terminal ends of the cords then form the acini of the glands.
- At around the 18th week of gestation, the parotid glands starts to secrete saliva via the parotid duct. In addition, the supporting connective tissue of the gland forms from the surrounding mesenchyme.
Vasculature of the parotid gland
- The parotid gland is supplied by the external carotid artery and its terminal branches within the gland, which are the superficial temporal and the maxillary artery, as well as the posterior auricular artery.
- The retromandibular veins drains the parotid gland.
- The preauricular or parotid lymph nodes drain the parotid gland, which then drain to the deep cervical chain.
Nerve supply of the parotid gland
- Sympathetic: The parotid gland is supplied by the cell bodies of the preganglionic sympathetic fibres, which are typically located in the lateral horns of upper thoracic spinal segments. Postganglionic sympathetic fibres from the superior cervical ganglion supplies the parotid gland by traversing along the external carotid artery and the middle meningeal artery.
- Parasympathetic: Preganglionic parasympathetic fibres supplying the parotid gland emerge from the brainstem in the inferior salivatory nucleus. They depart from the brain in the glossopharyngeal nerve (CN IX), then pass by the tympanic nerve to the tympanic plexus, and then from the tympanic plexus in the lesser petrosal nerve to the otic ganglion where they synapse. Postganglionic fibres from the ganglion then travel along the auriculotemporal nerve to approach the parotid gland.
- Sensory: The auriculotemporal nerve supplies the sensory innervation to the parotid gland and its capsule.
b. Submandibular glands
- The submandibular glands are major salivary glands located under the floor of the mouth. In adult humans, they weight about 15 grams and produce about 60-67% of saliva when unstimulated, but about decreases relative to the parotid gland secretion when stimulated.
- The average length of the submandibular salivary gland in an adult human is about 27 mm, and the average width is about 14.3 mm.
Describe the structure of the submandibular gland
- Each submandibular gland is split into a deep lobe and a superficial lobe, which are separated by the mylohyoid muscle.
- Saliva secretions enter the submandibular duct on the deep part after which they angle around the posterior edge of the mylohyoid muscle and traverse the superior surface laterally.
- The excretory ducts are subsequently traversed by the lingual nerve, and drain into the sublingual caruncles, which are small protuberances on either side of the lingual frenulum along with the major sublingual duct.
- The gland is bilaterally palpated inferior and posterior to the mandible, shifting inward from the inferior border of the mandible close to its angle with the head pitched forwards.
- The terminal aspect of the submandibular (Wharton's duct) is located in the mouth floor and spreads out as an orifice of the submandibular duct papilla.
- Anicin et al. (2023) used sialoendoscopy to describe 4 different types of submandibular gland papillae: types A, B, C, and D, based on the macroscopic appearance of the papillae.
Microanatomy of the submandibular gland
 | |
| This is a picture of a human submandibular gland under electron microscope. On the left is a group of serous acini, and on the right is a group of mucous acini. |
- The lobes contain smaller lobules, which contained the secretory units of the submandibular gland called adenomeres. Each ademoner contains at least one acini, or alveoli, which are small clusters of cells that release their products into a duct.
- The acini of each adenomere are composed of either serous or mucous cells. Some mucous adenomeres may have a layer of serous demilune, which secretes lysozyme.
- Submandibular glands are classified as branched tubuloacinar glands due to the branched glands and the tubules forming the branches consisting of secretory cells.
- Furthermore, the submandibular gland is a functionally mixed gland because it contains both serous and mucous secretory cells. In fact, a majority of those cells are serous, whereas the exudate is primarily mucous.
- A 2011 Elsevier article by Bath-Balogh and Fehrenbach described the submandibular gland having both short intercalated ducts and long striated ducts.
- The mucous cells of the submandibular glands produce saliva that is naturally mucoid. In addition, they release mucin to help lubricate the food bolus as it travels through the oesophagus. In contrast, the serous cells secrete salivary amylase to help disintegrate starches in the mouth.
Vasculature of the submandibular gland
- The facial and lingual arteries provide blood supply to the submandibular gland via the sublingual and submental arteries. In addition, the common facial and lingual veins drains blood away from the gland.
- The lymphatics from the submandibular gland initially drain into the submandibular lymph nodes and subsequently into jugulo - digastric lymph nodes.
Nerve supply of the submandibular gland
The secretions of this salivary gland are regulated directly by the parasympathetic nervous system and indirectly by the sympathetic nervous system.
- Parasympathetic nerve supply comes from the by the superior salivatory nucleus via the chorda tympani, which is a branch of the facial nerve that becomes a component of the trigeminal nerve's lingual nerve before synapsing on the submandibular ganglion. Moore et al. (2010) stated that increased parasympathetic nerve activity stimulates the secretion of saliva.
- Sympathetic innervation regulates saliva secretion from the submandibular gland by vasoconstriction of the arteries supplying it. Increased sympathetic activity decreases blood flow to the submandibular gland, therefore decreasing the volume of fluid in salivary secretions. This produces a mucous saliva full of enzymes. However, direct sympathetic innervation stimulates increase in salivary enzymatic secretions.
- Bruce Koeppen (2010) stated that both parasympathetic and sympathetic nerve stimulations decreases the volume of saliva, but increases the salivary secretions.
Describe the development of the submandibular gland
- The submandibular salivary glands develop later than the parotid glands around the 6th week of prenatal development. They develop bilaterally from epithelial buds in the sulcus that surrounds the sublingual folds on the floor of the primitive mouth.
- Solid cords split off from the buds and expand posteriorly, lateral to the developing tongue. The cords then branch further and become canalised to develop the ductal aspect.
- Development of the submandibular gland acini from the cords' rounded terminal completes at around 12 weeks. Secretory activity via the submandibular duct initiates around 4 weeks later.
- The submandibular gland continues to develop after birth with the creation of additional acini. A linear groove starts to form laterally to both sides of the tongue, which then closes over to create the submandibular duct.
Describe the function of the submandibular gland
- The submandibular gland secretes a diverse range of chemicals that regulate both systemic inflammatory responses and systemic immune and inflammatory reactions.
- Mathison et al. (1997) discovered a 7 amino acid peptide called the submandibular gland peptide-T (SGP-T) played an important role in the cervical sympathetic trunk-submandibular gland (CST-SMG) axis. SGP-T is found to elicit biological and thermoregulatory properties associated with endotoxin exposure.
- Furthermore, SGP-T is found to elicit immunoregulatory properties and play an important role in regulating the CST-SMG axis, as well as regulating inflammation.
c. Sublingual glands
- Also known as glandula sublingualis in Latin, the sublingual gland is described as a seromucous polystomatic exocrine gland. It is the smallest and most dispersed of the 3 major salivary glands, and provides roughly between 3 and 5% of the total salivary volume. It is located beneath the oral diaphragm (or diaphragma oris).
Describe the structure of the sublingual glands
- The sublingual glands are located anterior and superior to the submandibular gland and inferior and lateral to the tongue, as well as under the mucous membrane of the mouth floor. They are tethered laterally by the bone of the mandible and inferolaterally by the mylohyoid muscle.
- The sublingual gland composes of one major duct and roughly 20 small excretory ducts, with the latter referred to as the ducts of Rivinus.
- The sublingual duct (of Bartholin) connects to the submandibular duct to drain through the sublingual caruncle. The sublingual caruncle is a small papilla located adjacent to the midline of the mouth floor on each side of the lingual frenum.
- A majority of the remaining small sublingual ducts (of Rivinus) open separately into the mouth on an elevated crest of mucous membrane called the plica sublingualis (or sublingual fold). This fold is created by the gland and is situated on each side of the frenulum linguae.
- The sublingual gland contains mainly of mucous acini capped with serous demilunes, as well as striated and intercalated ducts.
- The sublingual and submental arteries provide blood to the sublingual gland. Lymph from the sublingual salivary gland drains into the submandibular lymph nodes.
1. The junction between pons and medulla
2. Via Internal acoustic meatus and facial canal to chorda tympani
3. Via the middle ear cavity
4. Exit through the petrotympanic fissure to link with the lingual nerve
5. Travels with lingual nerve to synapse at the submandibular ganglion
6. Postganglionic nerve fibres travels to the sublingual gland
- The sublingual salivary glands first appear around the 8th week of prenatal development. They develop from epithelial buds in the sulcus surrounding the sublingual folds on the mouth floor, which situates laterally to the developing submandibular gland.
- These buds split and form into cords that canalise to create the sublingual ducts linked to the gland, and the rounded terminal ends of the cords transform to become acini.
d. Tubarial salivary glands
- Valstar et al. (2020) used prostate-specific membrane antigen PET-CT to discover a 4th pair of salivary glands called the tubarial glands. They are located posteriorly in the nasopharynx and nasal cavity, mainly with mucous glands, and its ducts opening into the dorsolateral pharyngeal wall. However, more research is required to understand them and their functions.
e. Minor salivary glands
- It's estimated between 800 and 1000 minor salivary glands are positioned throughout the oral cavity within the submucosal of the oral mucosa. Moreover, they are located in the tissues of the buccal, labial, and lingual mucosa, the soft palate, the lateral sections of the hard palate, and the mouth floor or between muscle fibres of the tongue.
- These salivary glands typically contain a number of acini that are joined together in a tiny lobule, and a common excretory duct.
- Herring & Fehrenbach (2012) found the minor salivary glands are innervated by the 7th cranial nerve or facial nerve.
f. Von Ebner's glands
- Known as the gustatory glands, Von Ebner's glands are exocrine glands located in the mouth. Moreover, they are serous salivary glands that are located near the moats surrounding the circumvallate and foliate papillae anterior to the posterior 3rd of the tongue, which is anterior to the terminal sulcus. They are named after an Austrian histologist called Victor von Ebner.
- These glands release an enzyme called lingual lipase, which plays a role in lipid hydrolysis in the mouth.
- They drain their serous secretions into the base of the moats around the circumvallate and foliate papillae. It is thought this secretion rinses material from the mouth in order to allow a rapid response by the taste buds to varying stimuli.
- Von Ebner's glands are innervated by the glossopharyngeal nerve (CN IX).
What nerves supply the salivary glands?
- Salivary glands are innervated, either directly or indirectly, by the parasympathetic and sympathetic branches of the automatic nervous system.
- The glossopharyngeal nerve (CN IX) via the otic ganglion provides parasympathetic nerve supply to the parotid gland, while the facial nerve (CN VII) via the submandibular ganglion provides parasympathetic nerve supply to the submandibular and sublingual glands. These nerves release acetylcholine and substance P, which activate the IP3 and DAG pathways respectively.
- Sympathetic nerve supply to the salivary glands is provided by preganglionic nerves in the thoracic segments T1-T3 that synapse in the superior cervical ganglion with postganglionic neurons that secrete noradrenaline. β1-adrenergic receptors on the acinar and ductal cells of the salivary glands receives the noradrenaline, which stimulates an increase in cyclic adenosine monophosphate (cAMP) levels, therefore increases the release of saliva.
- It is interesting that both parasympathetic and sympathetic innervation lead to an increase in salivary gland secretions, with the major difference being the composition of the saliva being secreted.
- Sympathetic stimuli increases the secretion of saliva containing amylase by the serous glands, as well as indirectly decreasing blood flow through the activation of α1 adrenergic receptors, which decreases the amout of water in the saliva.
Microanatomy of the salivary glands
- The interior of the salivary gland is segmented into lobules, where blood vessels and nerves connect to via the hilum.
- A group of secretory cells that make up the salivary gland is called an acinus (plural: acini). Each acinus is at the terminal region of the gland linked to the ductal system, with numerous acini within each lobule of the gland. Each acinus contains a single layer of cuboidal epithelial cells surrounding the lumen, which is the main opening where saliva is settled after being produced by the secretory cells. Gilloteaux & Afolayan (2014) described 3 types of acini according to the type of epithelial cell and the secretory product being created, which are serous, mucoserous and mucous.
- The lumina are formed by intercalated ducts, which subsequently combine to form striated ducts. They drain into ducts located between the interlobular ducts or secretory ducts of the gland, which are situated on a majority of major and minor glands (except the sublingual gland). All of the human salivary glands end in the mouth, where saliva plays a role in the digestion process. The saliva is then rapidly inactivated by the acid in the stomach, however some enzymes within the saliva are activated by the same stomach acid.
Genes and protein expression
- Uhlén et al. (2015) estimated about 20,000 protein-coding genes are expressed in human cells and roughly 60% of these genes are expressed in normal, adult salivary glands. The most expressed salivary gland proteins are the heterogeneous family of proline-rich, human salivary glycoproteins, such as PRB1 and PRH1. Other specifically expressed proteins include the the digestive amylase enzyme AMY1A, the mucin MUC7 and statherin.
Describe the functions of salivary glands
- Protection = Saliva contains proteins such as mucins that lubricate and protect both the soft and hard tissues of the oral cavity. Tabak et al. (1982) stated that mucins are the primary organic ingredients of mucus, a slimy viscoelastic substance that coats all mucosal surfaces.
- Buffering = The higher the saliva flow rate, the faster the clearance and the higher the buffer capacity, therefore results in better protection from dental caries. Therefore, people with a reduced rate of saliva secretion, as well as a decreased buffer capacity, have reduced salivary protection against microbes.
- Formation of pellicles = Saliva creates pellicles on the tooth surface in order to avoid wearing. This layer consists of mucins and proline-rich glycoprotein provided by the saliva.
The proteins, e.g. statherin and proline-rich proteins, situated within the salivary pellicle inhibit demineralisation and promote remineralisation by attracting calcium (Ca(2+)) ions.
- Maintaining tooth integrity = Demineralisation occurs when enamel disintegrates after interacting with acid. Saliva flow increases to create a buffering effect in order to inhibit demineralisation. Saliva can subsequently initiate the remineralisation of the tooth by strengthening the enamel with calcium and phosphate.
- Antimicrobial effects = Saliva contains molecules that prevent the growth of microbes. For example, lactoferrin binds naturally with iron that breaks down the bacterial cell wall, thus disintegrates the bacterium. Antimicrobial peptides such as histatins inhibit the growth of Candida albicans and Streptococcus mutans. Salivary immunoglobulin A can aggregate oral bacteria such as S. mutans and prevent the development of dental plaque.
- Tissue repair = Saliva promote repair of soft tissue by reducing clotting time and increasing wound contraction.
- Digestion = Saliva contains an enzyme called amylase that hydrolyses starch into dextrin, glucose and maltose. This triggers the digestion process prior to the food approaching the stomach.
- Taste = Saliva serves as a solvent that dissolves solid particles, which allows them to interact with the taste buds through oral mucosa located on the tongue. These taste buds are located within foliate and circumvallate papillae, which minor salivary glands release saliva.
3. Saliva
- Commonly referred to as spit, saliva is an extracellular fluid created and released by salivary glands in the mouth.
- There is debate regarding the amount of saliva produced by a healthy human. Edgar, Dawes & O'Mullane (2004) estimated humans produce about 1500 mL of saliva per day and that number readily decreases during sleep.
- A 2018 Cedars-Sinai article estimated the submandibular contributes roughly 70-75% of salivary secretions, whereas the parotid gland contributes about 20-25% of salivary secretions, with the remaining amount released from other salivary glands.
What is saliva composed of?
Human saliva is composed of 99.5% water, which the remaining 0.5% comprising of numerous molecules, such as antibacterial substances, mucus, electrolytes, and enzymes.
- Water: 99.5%
- Electrolytes:
- Sodium = 2 - 21 mmol/L
- Potassium = 10 - 36 mmol/L
- Calcium = 1.2 - 2.8 mmol/L
- Magnesium = 0.08 - 0.5 mmol/L
- Chloride = 5 - 40 mmol/L
- Bicarbonate = 25 mmol/L
- Phosphate = 1.4 - 39 mmol/L
- Iodine = Levels typically higher than plasma, but it varies depending on dietary iodine intake
- Mucus = Consists of mucopolysaccharides and glycoproteins
- Antibacterial compounds = Hydrogen peroxide, thiocyanate, and secretory immunoglobulin A
- Epidermal growth factor (EGF)
- Enzymes:
- α-amylase (EC3.2.1.1), or ptyalin = Secreted by the acinar cells of the parotid and submandibular glands. It initiates the digestion of starch before the food is swallowed. Its optimal pH is around 7.4.
- Lingual lipase = Secreted by the acinar cells of the sublingual gland. Its optimal pH is around 4.0 so it doesn't get activated until it enters the acidic environment of the stomach.
- Kallikrein = Proteolytically cleaves kininogen to yield a vasodilator called bradykinin. It is secreted by the acinar cells of all 3 major salivary glands.
- Antimicrobial enzymes = Lysozyme, salivary lactoperoxidase, lactoferrin, immunoglobulin A
- Proline-rich proteins = Play important roles in development of enamel, binding to Ca2+ ions, killing microbes and lubrication
- Minor enzymes = Salivary acid phosphatases A+B, N-acetylmuramoyl-L-alanine amidase, NAD(P)H dehydrogenase (quinose), superoxide dismutase, glutathione transferase, class 3 aldehyde dehydrogenase, glucose-6-phosphate isomerase, and tissue kallikrein.
- Cells = Estimated to be around 8 million human and 500 million bacterial cells per mL of saliva. Saliva may contain bacterial products such as amines, thiols and small organic acids that can release a foul stench.
- Opiorphin = This molecule eliminates pain.
- Haptocorrin = This protein binds to vitamin B12 to protect it against degradation in the stomach, prior to its binding to intrinsic factor.
Describe the functions of saliva
- Lubrication = Saliva coats the oral mucosa to protect it from strain during eating, swallowing, and speaking.
- Digestion = Moistens food to produce a food bolus that allows it to move from the mouth into the oesophagus easily. Saliva contains amylase (ptyalin) that disintegrates starch into smaller sugars such as dextrin and maltose, which can be further disintegrated in the small intestine. Saliva also contains salivary lipase that aids in the digestion of fat.
- Taste = Saliva carries food molecules to the taste receptor cells that are mainly associated with lingual papillae.
- Saliva is supersaturated with various ions (formed by certain salivary proteins that prevent precipitation) acting as a buffer to maintain the aciditiy of the mouth between 6.2 and 7.4 on pH rating. This protects minerals in the dental hard tissues from dissolving.
- Contains carbonic anhydrase (gustin) to support development of taste buds.
- Contains epidermal growth factor (EGF) to promote cellular proliferation, differentiation, and survival, as well as maintain oro-oesophageal and gastric tissue integrity. This results in healing of oral and gastrooesophageal ulcers, inhibition of gastric secretion, stimulation of DNA synthesis and mucosal protection from intraluminal injurious factors such as gastric acid, bile acids, pepsin, and trypsin and to physical, chemical and bacterial agents.
- Inhibits growth of bacterial pathogens and maintains systemtic and oral health by preventing tooth decay, eliminating sugars and other food sources of microbes.
4. What is a tongue?
The tongue is an organ inside the mouth that tastes the food and plays a role in both chewing and swallowing processes. The word tongue originates from the Old English word tunge, which derives from the Proto-Germanic word *tungōn. The Online Etymology Dictionary suggested the ue- suffix was added around the 14th century as an attempt to indicate its "proper pronunciation", but it is neither "etymological or phonetic".
How does the tongue develop?
- The tongue's development starts in the 4th week of embryonic development from a median swelling called the median tongue bud (tuberculum impar) of the first pharyngeal arch.
- During the 5th week of development, a pair of lateral lingual swellings appear on both the left and right sides of the first pharyngeal arch. They subsequently expand and cover the median tongue bud.
- This creates the anterior area of the tongue that comprises 2/3 of the tongue's length, which continues developing through prenatal stage of development. The median sulcus demarcates the line of their fusion.
- During week 4 of development, a swelling emerges from the second pharyngeal arch located in the midline called the the copula.
- During weeks 5 and 6 of development, a swelling from the 3rd and 4th arches called the hypoharyngeal eminence overgrows the copula, which then develops into the posterior area of the tongue. Moreover, the other 3rd and most posterior area of the tongue is developed from the 4th pharyngeal arch. The hypopharyngeal eminence develops by the endodermal growth from the 3rd pharyngeal arch.
- In 2001, William Larsen discovered a V-shaped groove called the terminal sulcus marks the boundary between the anterior and posterior aspects of the tongue.
- Drake, Vogl & Mitchell (2005) described the foramen caecum being at the tip of the terminal sulcus, where the thyroglossal duct connects to and the embryonic thyroid starts to descend.
Describe the structure of the tongue
- The tongue is a muscular hydrostat that is attached to the floor of the oral cavity. A vertical section of fibrous tissue called the lingual septum separates the left and right sides of the tongue.
- The division is situated along the length of the tongue except for the rear of the pharyngeal area, which is a groove called the median sulcus.
- A V-shaped groove called the terminal sulcus divides the human tongue into anterior and posterior halves.
- A remnant of the median thyroid diverticulum in early embryonic development called the foramen caecum forms the apex of the terminal sulcus.
- Approximately 2/3 of the length of the tongue is the anterior oral component, which is visible at the front. On the other hand, 1/3 of the length of the tongue is the posterior pharyngeal component, which is situated closest to the throat.
- Each part develop in different ways embryologically and receive different sources of nerve supply. The anterior part is located at its apex that directs forward against the lingual surfaces of the lower incisor teeth.
- The posterior part is located at its root and is directed backward, and is linked with the hyoid bone by the hyoglossi and genioglossi muscles and the hyoglossal membrane. It is also linked with the epiglottis by 3 glossoepiglottic folds of mucous membrane, with the soft palate by the glossopalatine arches, and with the pharynx by the superior pharyngeal constrictor muscle and the mucous membrane. Furthermore, it forms part of the anterior wall of the oropharynx.
- In 1997, Robin Kerrod measured the average length of the human tongue from the oropharnyx to the tip is about 10 cm.
- In phonetics and phonology, sounds made with the tip of the tongue are apical, whereas sounds made with the tongue blade (just behind the tip) are laminal.
i. Upper surface
- The tongue's upper surface is referred to the dorsum, which is split by a groove into symmetrical halves by the median sulcus. The foramen caecum marks the end of this division (~2.5 cm from the tongue's root) and the start of the terminal sulcus. Moreover, it connects to the thyroglossal duct and forms during the descent of the thyroid diverticulum in embryonic development.
- The terminal sulcus is a shallow groove in a V-shape formation running forwards and outwards from the foramen caecum to the edges of the tongue.
- The glossopharyngeal nerve innervates the pharyngeal section, and the lingual nerve (a nerve of the mandibular branch (V3) of the trigeminal nerve) and the chorda tympani (a branch of the facial nerve) innervates the oral section for somatosensory perception and taste perception respectively.
ii. Undersurface
- The tongue's undersurface contains a fold of mucous membrane called the frenulum, which tethers the tongue at the midline to the mouth floor.
- There are small protuberances on either side of the frenulum called sublingual caruncles, which drain the major salivary submandibular glands.
What muscles control the tongue?
There are a total of 8 muscles that control the human tongue. 4 of them are intrinsic that manipulate the shape of the tongue, and aren't attached to any bone. Thus the other 4 muscles are extrinsic, which manipulate the tongue's position, and are anchored to bone.
i. Intrinsic
- The 4 paired intrinsic muscles of the tongue originate and enter within the tongue, which continue along its length.
- They include the superior longitudinal muscle, the inferior longitudinal muscle, the vertical muscle, and the transverse muscle.
- They can manipulate the tongue's shape by shortening and lengthening it, curling and uncurling its apex and edges i.e. tongue rolling, and flattening and rounding its surface. It provides important functions such as eating, facilitating speech, and swallowing.
- The superior longitudinal muscle stretches along the tongue's upper surface under the mucous membrane, and rises up, supports in retraction of, or deviation of the tip of the tongue. It originates close to the epiglottis, at the hyoid bone, from the median fibrous septum.
- The inferior longitudinal muscle lines the sides of the tongue, and connects to the styloglossus muscle.
- The vertical muscle is situated in the middle of the tongue, and connects to the superior and inferior longitudinal muscles.
- The transverse muscle bisects the tongue at the middle, and is connected to the mucous membranes that stretch along the sides.
ii. Extrinsic
- The 4 extrinsic muscles originate from the bone and continue on to the tongue. They are the genioglossus, the hyoglossus (inc. the chondroglossus), the styloglossus, and the palatoglossus.
- Their main functions include changing the tongue's position in order for protrusion, retraction, and lateral movement to occur.
- The genioglossus originates from the mandible and protrudes the tongue, which is regarded as the tongue's "safety muscle" since it is the only muscle that drives the tongue in a forward direction.
- The hyoglossus originates from the hyoid bone, then retracts and depresses the tongue.
- The styloglossus originates from the styloid process of the temporal bone and lifts the sides of the tongue upwards to produce a trough for swallowing.
- The palatoglossus originates from the palatine aponeurosis, and depresses the soft palate, which shifts the palatoglossal fold towards the midline, and lifts the back of the tongue during swallowing.
Tongue's blood supply
- A branch of external carotid artery called the lingual artery supplies blood to the tongue, as well as the mouth floor. In addition, the lingual veins drain into the internal jugular vein.
- A secondary blood supply to the root of the tongue originates from the tonsillar branch of the facial artery and the ascending pharyngeal artery.
- Located in the neck is the Pirogov triangle, which is formed by the intermediate tendon of the digastric muscle, the posterior border of the mylohyoid muscle, and the hypoglossal nerve.
Tongue's nerve supply
The tongue is innervated by a combination of motor fibres, general sensory fibres for sensation, and special sensory fibres for taste.
- Efferent motor nerve fibres from the hypoglossal nerve (CN XII) innervates all of the tongue's intrinsic and extrinsic muscles, except for the palatoglossus, which is innervated by the vagus nerve (CN X).
- For the anterior 2/3rds of the tongue (anterior to the vallate papillae), taste perception is provided by the chorda tympani branch of the facial nerve (CN VII) via special visceral afferent fibres, and general sensation is provided by the lingual branch of the mandibular (V3) division of the trigeminal nerve (CN V) via general visceral afferent fibres.
- For the posterior 1/3rd of the tongue, both taste and sensation is provided by the glossopharyngeal nerve (CN IX) via a combination of special and general visceral afferent fibres.
- For the base of the tongue, taste and sensation is provided by the internal branch of the superior laryngeal nerve, which itself is a branch of the vagus nerve (CN X).
- Dr Donald Runek (2014) stated the reason for the differences in innervation of taste and sensation for the anterior and posterior aspects of the tongue is the derivation from different embryological structures (pharyngeal arch 1 and pharyngeal arches 3 and 4, respectively).
Tongue's lymphatic drainage
- The tip of the tongue drains to the submental nodes.
- Both halves of the anterior 2/3rds of the tongue drains to submandibular lymph nodes, whereas the posterior 1/3rd of the tongue drains to the jugulo-omohyoid nodes.
Describe the microanatomy of the tongue
- The tongue's upper surface has a layer of oral mucosa called masticatory mucosa, which is keratinised stratified squamous epithelium. There are papillae submerged in this oral mucosa, some of which contain the taste buds and their taste receptors.
- The lingual papillae contains filiform, fungiform, foliate and valiate papillae. However, only the filiform papillae have no relationship with any taste buds.
- Fiore & Eroschenko (2000) described the tongue's dorsal surface as a stratified squamous keratinised epithelium, which is denoted by many mucosal projections known as papillae. In addition, the lingual papillae is embedded on the tongue's dorsal side towards the front of the terminal groove.
- Hib (2001) described the tongue's ventral surface as a stratified squamous non-keratinised epithelium, which appears smooth.
What are the functions of the tongue?
i. Taste
- Chemicals that enter the mouth and interact with taste receptor cells are called tastants. Saliva dissolves the tastant(s) into smaller particles, which allow them to bind with the plasma membrance of the gustatory hairs, where taste transduction occurs.
- The tongue's dorsal surface comprises of numerous taste buds, and each taste bud contains taste receptor cells that perceive different tastes such as bitter, sweet, sour, salty, spicy or umami.
- A common misconception about the tongue is that each basic taste are exclusively perceived by different regions of the tongue, which is illustrated by a tongue map.
- The fact is all taste sensations is detected on all regions of tongue, although particular areas of the tongue are more sensitive to particular tastes.
ii. Mastication (Chewing)
- During the mastication and manipulation of food, the tongue squashes food against the hard palate in order to soften it before it gets swallowed.
- Since the tongue's dorsal surface has keratinised epithelium, it can crush against the hard palate without damaging or irritating its structure.
iii. Speech
- The tongue is one of the main articulators in speech production, which is facilitated by both the extrinsic muscles that control the tongue 's movement and the intrinsic muscles that control the tongue's shape.
- When the tongue's height changes and its position retracts to change the resonant characteristics of the vocal tract, it articulates different vowels. The resonant features amplify certain harmonic frequencies for each unique vowel, while reducing other harmonics.
- The tongue moves down and towards the centre to produce the letter 'a', whereas it moves up and towards the front to produce the letter 'i'.
- Tongue shape plays a role in producing retroflex consonants, where the tip of the tongue curves backward.
- I'll discuss how the voice and the process of speaking works in another post.
iv. Intimacy
The tongue is part of the mouth's erogenous zone and is involved in situations of intimate contact, such as the French kiss and oral sex. It also plays a role in stimulating the clitoris and other parts of the vulva. I'll discuss sexual intimacy in another post.
What is a taste bud?
- The taste buds situated on the tongue are located on raised protrusions of the tongue surface called papillae. They are also found on the soft palate, the cheek, epiglottis and the upper oesophagus.
- Taste buds contain gustatory cells or taste receptor cells that detect the 5 main tastes: bitterness, saltiness, sourness, sweetness, and umami.
- The tiny openings in the tongue epithelium are called taste pores, where food particles dissolved in saliva interact with receptors. They are found on top of the taste receptor cells that comprise the taste buds.
- The gustatory cells transmi taste information perceived by numerous receptors and ion channels to the gustatory regions of the brain via CN7, CN9 and CN10.
- The human tongue contains an average of 2000 - 8000 taste buds, with each taste bud turning over after 10 days on average.
What are the different types of papillae?
- Lingual papillae are rough, small structures situated on the tongue's upper surface. There are 4 types of lingual papillae, which as circumvallate, filiform, foliate and fungiform.
- The word 'lingual' originates from the Latin word lingua meaning "tongue" or "speech", whereas papilla is derived from the Latin word for 'nipple'.
- Vallate is derived from the Latin word vallum (wall, rampart), which is defined as "having a raised edge surrounding a depression". This describes the circular mucosal elevation surrounding the circumvallate papillae.
- Fungiform is derived from the Latin words fungus (mushroom) and forma, which means "shaped like a mushroom or fungus".
- Foliate is derived from the Latin word foliatus (leafy), which means "shaped like a leaf".
- Filiform is derived from the Latin word filum (thread), which means "shaped like a filament or thread".
i. Circumvallate papillae
- Sometimes referred to as vallate papillae, circumvallate papillae are dome-shaped structures on the human tongue that can range in number from 8 to 12.
- They are located on the tongue's surface immediately ahead of the foramen caecum and sulcus terminalis, which creates a row on either side. Both rows stretch backward and medially, and join in the midline.
- Each papillae protrudes from the mucous membrane with a width between 1 and 2 mm that connects to the bottom of a circular depression of the mucous membrane.
- The depression's margin rises up to create a wall (vallum), and the circular sulcus formed between the wall and the papilla is called the fossa.
- It is shaped like a truncated cone, with the smaller end heading downward and connecting to the tongue. On the other hand, the larger end or base directing slightly above the tongue's surface and becoming scattered with scores of small secondary papillae and enveloped by stratified squamous epithelium.
- The ducts of lingual salivary glands, called Van Ebner's glands, secrete a serous substance into the base of the circular depression. This substance washes materials from there to ensure that taste buds are able to respond to different stimuli quickly.
- The circumvallate papillae receives afferent taste innervation from CN IX or the glossopharyngeal nerve, despite being situated anterior to the sulcus terminalis.
- The remaining anterior 2/3rds of the tongue receives taste innervation from the chorda tympani of CN VII, which is distributed with the the lingual nerve of CN V.
ii. Filiform papillae
- They are the most abundant of the lingual papillae. They are described as fine, little, cone-shaped papillae situated on the tongue's front 2/3rds anterior surface.
- They are responsible for the tongue's texture and sensation of touch.
- Unlike other types of papillae, filiform papillae don't contain any taste buds.
- They appear as microscopic, conical or cylindrical surface protrusions, organised into rows lying parallel to the sulcus terminalis. Those rows become more transverse at the tip of the tongue.
- Histologically, they consist of asymmetrical connective tissue cores with an epithelium that contains keratin, which gives the appearance of fine secondary threads.
- Due to the density and thickness of the epithelium, these papillae appear slightly white. As the taste cells becomes more conical and elongated into dense, overlapping, brush-like threads, the epithelium undergoes a modification.
- In addition, the papillae consist of elastic fibres that enhance its firmness and elasticity.
iii. Fungiform papillae
- These papillae are club-shaped protrusions on the tongue, which appear red in colour. They are mostly located on the tip and sides of the tongue, which are dispersed amongst the filiform papillae.
- These contain taste buds on their upper surface that is capable to distinguish the 5 main tastes: bitter, salty, sour, sweet, and umami.
- They are innervated by CN XII, specifically via the submandibular ganglion, chorda tympani, and geniculate ganglion ascending to the solitary nucleus in the brainstem.
iv. Foliate papillae
- These papillae are tiny, bilaterally symmetrical vertical folds with variable size and shape, which appear as a set of red leaf-like folds of mucosa.
- They are located on both sides of the tongue and at the back, in front of the palatoglossal arch of the fauces.
- They are covered with a layer of epithelium, but lack keratin, meaning they are softer and contain numerous taste buds.
- This papillae's location is a high risk site for oral cancer, and swelling at this region may be misidentified as inflammatory disease or tumours.
- Lingual tonsils are located immediately behind the foliate papillae, and they increase the papillae projections when they're hyperplastic.
Describe the cell composition of taste buds
Taste buds are produced by 2 types of cells: gustatory cells and supporting cells.
- Known as sustentacular cells, the supporting cells are mainly organised like staves of a cask, which form an outer envelope for the taste bud. Some supporting cells may be found inside the taste bud between the gustatory cells.
- The gustatory (taste) cells are spindle-shaped chemoreceptors containing a large spherical nucleus close to the middle of the cell that are situated in the central part of the taste bud. The peripheral end of the taste cell ends at the gustatory pore in a fine hair filament called the gustatory hair. The central process crosses toward the deep end of the bud, which then become single or bifurcated varicosities.
- After losing their medullary sheaths, the nerve fibrils enter the taste bud, and terminate in the boundaries between the gustatory cells. Other nerve fibrils furcate between the supporting cells and end in fine borders, however they may be nerves of ordinary sensation rather than gustatory.
5. What is taste?
- You're feeling hungry and you want to try out a new food that you haven't eaten before. But you feel unsure about its flavour(s), so you naturally want to smell or taste it to gain a sense of the flavours.
- Taste is defined as a sensation when food in the mouth reacts chemically with taste receptor cells situated on taste buds in the oral cavity, primarily on the tongue and the epiglottis. Your perception of taste is sensed by the gustatory system.
- Gustation is combined with olfaction and trigeminal nerve stimulation (i.e. perception of pain, texture, and temperature) to determine the flavours of the food and other substances being consumed.
What are different types of taste?
- Animals have gustatory systems that help them distinguish food that is safe to eat and food that is harmful to eat, as well as ascertain the foods' nutritional value.
- When food enters your mouth, it is dissolved into base chemicals by digestive enzymes in the saliva. Those base chemicals are subsequently washed over the papillae and perceived as distinct tastes by the taste buds.
- The tongue's surface contains 1000s of small bumps called papillae, with each papilla home to 100s of taste buds. However, one exception are the filiform papillae, which don't contain any taste buds.
- Boron & Boulpaep (2003) estimated between 2000 and 5000 taste buds are located on the back and front of the tongue, and more taste buds located on the roof, sides and back of the mouth, and in the throat. Each taste bud roughly contains between 50 and 100 tate receptor cells.
A. Bitterness
 |
| https://www.researchgate.net/figure/Signal-transduction-pathway-of-bitter-T2R-sweet-T1R2-3-and-umami-T1R1-3-GPCRs-in_fig1_267043464 |
- Bitter foods are primarily perceived as sharp, nasty, or unpleasant, but some may perceive as desirable and intentionally add bitter flavours using bittering agents. The chemical responsible for the bitter taste is quinine, which is usually found in tonic water.
- Common bitter foods include: Lemon, citrus peel, gourd, uncured olives, some cheeses, a number of plants in the Brassicaceae family, dandelion greens, horehound, wild chicory, and escarole.
- Common bitter beverages include: coffee, raw cocoa, South American mate, coca tea, some alcoholic beverages due to ethanol content and additional bitter ingredients such as hops in beer and gentian in bitters.
- Researchers stated a considerable number of compounds that are naturally bitter are known to be toxic. Guyton (1991) regarded the ability to perceive toxic compounds as bitter at low thresholds as a protective mechanism.
- Jones, Martin & Pilbeam (1994) found mature plant leaves tend to contain toxic compounds, meaning primates tend to eat immature leaves that have higher protein content and lower fibre and toxic content.
- Johns (1990) stated several food processing techniques allow humans to detoxify foods that are usually inedible in order to make them palatable. Moreover, diet changes, the use of fire, and avoidance of toxins resulted in neutral evolution in human bitter sensitivity. Wang (2004) found this resulted in a number of loss-of-function mutations that decreased sensory capacity towards bitterness in humans compared to other species.
- Guyton (1991) calculated the average concentration threshold for stimulation of bitter taste by quinine is about 8 μM (8 micromolar). McLaughlin & Margolskee (1994) rated the taste thresholds of other bitter substances relative to quinine, which is specified as a reference index of 1.
- e.g. Brucine has an index of 11, extremely more bitter than quinine, thus is detected at a significantly lower threshold.
- The most bitter natural substance is a compound found in the roots of the plant Gentiana lutea called amarogentin.
- With an index of 1000, the most bitter substance known is the synthetic chemical denatonium. This aversive agent (bitterant) is usually added to toxic substances to avoid accidental ingestion. It was accidentally discovered in 1958 by Scottish research MacFarlan Smith of Gorgie, Edinburgh during research on a local anesthetic.
- Maehashi et al. (2008) demonstrated that TAS2Rs (taste receptors, type 2, also known as T2Rs), such as TAS2R38 linked to the G protein gustducin give humans the ability to taste bitter compounds.
- Lindemann (2001) identified TAS2Rs receptors not only by its function to taste for specific "bitter" ligands, but also by the morphology of the receptor itself (i.e. monomeric, surface-bound).
- Meyerhof (2010) suggested the TAS2R family in humans contains about 25 unique taste receptors, with a number of them able to identify a broad range of bitter-tasting compounds.
- Wiener (2012) reported from a database that over 670 bitter-tasting compounds have been identified, of which over 200 are associated with 1 or more specific receptors.
- Wang, Thomas, & Zhang (2004) hypothesised that the selective constraints on the TAS2R family were diminished because of the relatively high mutation rate and pseudogenisation.
- In a study involving 2 synthetic substances, phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP), Wooding et al. (2004) discovered the 2 common alleles at the TAS2R38 locus influence the variation in sensitivity to bitter tastes.
B. Saltiness
- Salt taste is perceived by the binding of sodium ions onto the sodium chloride (salt) receptor. The sodium ion channel is called an epithelial sodim channel (ENaC) and consists of 3 subunits.
- When sodium (Na+) cations enters the taste cell via the sodium channel. This depolarises the cell and opens voltage-dependent calcium channels, allowing calcium cations to enter the taste cell, resulting in the release of neurotransmitters.
- Other ions of alkali metals group can also be perceived as salty, but less salty than sodium. Lithium and potassium ions are similar in size to sodium ions, therefore they have similar salt taste.
- Arthur Guyton (1991) described the saltiness of compounds is rated relative to sodium chloride (NaCl), which has an index of 1. For example, potassium chloride (KCl), the main ingredient in salt substitutes, has a saltiness index of 0.6.
- Other monovalent cations, such as ammonium (NH4+), and divalent cations of alkali metals, such as calcium (Ca2+) ions, taste bitter rather than salty, despite passing directly through ion channels of the taste cells in the tongue, resulting in the generation of action potential.
C. Sourness
- Substances that taste sour indicate the presence of acid. The sourness of compounds is rated relative to dilute hydrochloric acid, which has a sourness index of 1. In comparison, tartaric acid has an index 0.7, citric acid has an index of 0.46, and carbonic acid has an index of 0.06.
- Type III taste receptor cells detect protons (H+ ions) in sour substances by directly entering proton channels called otopetrin 1 (OTOP1). The influx of positive charges into the taste cell would result in the generation of an electrical response.
- Ye et al. (2016) found the combination of direct influx of protons through OTOP1 ion channels (depolarising the taste cell) and the suppression of the hyperpolarising potassium channel results in the taste cell to fire action potentials and release neurotransmitter to communicate the sourness taste information.
- Common sour foods include fruits such as grapes, lemons, limes, oranges, tamarinds and bitter melons, as well as fermented foods such as vinegar, wine, or yogurt.
D. Sweetness
- Foods rich in sugar or chemicals that resemble sugar are commonly perceived as sweet, which are considered delightful. In addition to sugars such as glucose, sucrose, fructose and galactose, other substances perceived as sweet include aldehydes, ketones, and sugar alcohols.
- In a solution, sucrose has a sweetness rating of 1, and other sweet substances are related to sucrose. e.g. Fructose is rated about 1.7 times sweeter than sucrose. Amino acids such as alanine, glycine and serine are perceived to be sweet.
- Several plant species produce glycosides that are as sweet as common sugars at significantly lower concentrations. Licorice root produces a sweet compound called glycyrrhizin that is about 30 times sweeter than sucrose. The South American shrub Stevia rebaudiana is estimated to be 250 times sweeter than sucrose.
- Potent natural sweeteners can also be sweet proteins such as thaumatin (discovered in the West African katemfe fruit) and hen egg lysozyme (a sweet antibiotic protein discovered in chicken eggs).
Sweetness of various molecules
- Note the variations in sweetness indexes is influenced by a range of methodological variables, such as sampling, analysis, and interpretation. Examples of sweet inorganic compounds include lead (II) acetate and beryllium chloride.
- A number of synthetic organic compounds known to be sweet include: acesulfame potassium, alitame, aspartame, chloroform, cyclamate, neotame, nitrobenzene, ethylene glycol, saccharin, sucralose.
What are sweetness modifiers?
- Sweet modifiers are chemical compounds that change the way our sweet taste is perceived. One type of sweet modifiers inhibit the perception of sweet tastes, whether from sugars or from highly potent sweeteners.
- An example of such compound is lactisole, produced by Domino Sugar, which is used in certain jellies and other fruit preserves that release the fruit flavours by suppressing their usual strong sweet flavours.
- Gymnemic acid is a natural compound, produced by the leaves of the Indian vine Gymnema sylvestre, that is known to inhibit the sweetness taste.
- Another natural product found to inhibit sweetness aste is ziziphin, which is produced by the leaves of Chinese jujube (Ziziphus jujuba).
- Miraculin and curculin are plant proteins that make sour foods taste sweet for a certain period of time.
Describe the sweetness receptor
- In 2001, mice studies discovered different versions of T1R3 gene preferring sweet foods to varying extents. Moreover, Li et al. (2002) discovered the T1R3 protein forms a complex with an associated protein called T1R2 to produce a G-protein coupled receptor to become the sweetness receptor in mammals.
- In 2017, Daisuke Kohno demonstrated sweet taste receptors are not only located in the tongue, but as well as in the lining of the gastrointestinal tract (GIT), nasal epithelium, pancreatic islet cells, sperm and testes. It is suggested the presence of sweet taste receptors in the GIT regulates the emotion of hunger and satiety.
- Nakamura et al. (2008) demonstrated the threshold of sweet taste perception is directly associated with the time of day. Researchers suggested this may be a result of oscillating leptin levels in the blood that may influence the overall sweetness of food. Another theory proposed is this may be an evolutionary artefact of diurnal animals such as humans.
- Nofre, Tinti & Glaser (1995) found New World monkeys are unable to perceive the sweetness of aspartame, whereas Old World monkeys and apes (including most humans) do. David Biello (2007) found felids such as domestic cats are unable to perceive sweetness at all.
- This variation in sweetness perception suggests genetic atrophy in the carnivores' ability to taste sweet flavours who usually don't eat sweet foods, which includes bottlenose dolphins, sea lions, spotted hyenas and fossas.
Describe the sweet receptor pathway
- Sweet molecules bind to the sweet taste receptors, which triggers a conformational change in the molecule.
- This activates a G-protein called gustducin, which subsequently activates phospholipase C to generate inositol trisphosphate (IP3).
- IP3 then opens the IP3-receptor and triggers calcium (Ca2+) release from the endoplasmic reticulum.
- This elevation in intracellular calcium activates the TRPM5 channel and triggers cellular depolarisation.
- The depolarised taste cell activates the channel CALHM1, which releases ATP neurotransmitter to activate the afferent neurons innervating the taste bud.
When were sweetness taste theories first proposed?
- In 1914, German chemist Georg Cohn made the first attempt to make systematic associations between the structures of molecules and their tastes.
- Cohn conjectured that a molecule had to contain a particular structural motif (i.e. a sapophore) in order to induce a particular taste. In the context of sweetness, he found molecules that contained several hydroxyl groups or chlorine atoms are perceived to be sweet. Furthermore, among a series of molecules with similar compounds, lighter molecules tend to be sweeter than heavier molecules.
- In 1919, Oertly and Myers hypothesised a sweet compound had to contain 1 of each 2 classes of structural motif a glucophore and an auxogluc. Based on this model, they suggested a list of 6 candidate glucophores and 9 auxoglucs.
- In 1963, Robert Shallenberger and Terry Acree proposed the AH-B theory of sweetness, which suggested a swet compound had to contain a hydrogen bond donor (AH) and a Lewis base (B) separated by about 0.3 nanometres. Based on this theory, the AH-B unit of a sweetener binds with a corresponding AH-B unit on the biological sweetness receptor to create the sweet taste.
- In 1972, Lemont Kier conjectured the B-X theory that suggested a compound has to contain a 3rd binding site (X) that can interact with a hydrophobic site on the sweetness receptor via London dispersion forces.
E. Umami
- Umami is described as savoury taste and is characteristic of broths and cooked meats. Umami is a Japanese word (うま味), which means "pleasant savoury taste".
- This term was coined by Japanese chemist Kikunae Ikeda in 1908 from a nominalisation of the Japanese word umai (うまい) meaning "delicious".
- This is combined with term mi (味) meaning "taste", which is used in the phrase 旨味 to generally describe food as "delicious".
- There is no current English equivalent of umami, however terms such as "broth-like", "savoury" and "meaty" are the closest in definition.
How was the notion of umami conceived?
- In 1985, the term umami was recognised as a scientific term to describe the taste of glutamates and nucleotides at the first Umami International Symposium in Hawaii.
- Yamaguchi & Ninomiya (2000) stated that umami describes the taste of the amino acid L-glutamate and 5'-ribonucleotides such as guanosine monophosphate (GMP) and inosine monophosphate (IMP).
- Beauchamp (2009) thought the umami flavours enhances the palatability of foods by balancing the taste and rounding out the overall flavour of the dish. This gives off a pleasant "brothy" or "meaty" taste with an enduring and mouthwatering sensation over the tongue.
- Zhang et al. estimated more than 50 peptides may play a role in perceiving umami taste. Finger (2009) added that the carboxylate anion of glutamate binding to specialised receptor cells on the tongue triggers the perception of umami. Studies found glutamic acid induces minimal umami taste, whereas glutamates (salts of glutamic acid) trigger the authentic umami taste due to their ionised state.
- Lioe et al. (2010) found the addition of salt, GMP or IMP to the free acids can enhance the umami taste. Furthermore, the umami taste of monosodium L-aspartate was perceived to be about 4 times less intense than monosodium glutamate (MSG) whereas the umami tastes of ibotenic acid and tricholomic acid are thought to be more intense.
How was umami discovered?
- During ancient times, high glutamate foods such as fermented fish sauces (garum), fermented barley saurces (murri) and fermented soy sauces were commonly used in ancient Rome, medieval Byzantine and Arab cuisine, and (3rd century) Chinese cuisine respectively.
- In the late 1800s, French chef Auguste Escoffier cooked up dishes containing bitter, salty, sweet, sour, as well as umami flavours. However, he didn't know the chemical nature of the umami flavour.
- In 1908, a professor of the Tokyo Imperial University named Kikunae Ikeda was the first to scientifically discover umami. He identified glutamate played a role in the palatability of the broth from kombu seaweed. He perceived the taste of kombu dashi was different from bitter, salty, sour, and sweet, and thus named it umami.
- In 1913, a disciple of Ikeda named, professor Shintaro Kodama, a disciple of Ikeda, discovered that dried bonito flakes (a type of tuna) contained another umami substance called IMP, a ribonucleotide.
- In 1957, Akira Kuninaka discovered the ribonucleotide GMP found in shiitake mushrooms also induced the umami sensation. Moreover, Kuninaka discovered the synergistic effect between ribonucleotides and glutamate. This means foods containing glutamate that are combined with ingredients containing ribonucleotides would greatly enhance the taste intensity.
-- Kombu seaweed and dried bonito flakes (Japanese)
-- Chinese leek and Chinese cabbage to chicken soup
-- Cock-a-leekie soup (Scottish)
-- Grated Parmigiano-Reggiano cheese on a variety of Italian dishes
What are the properties of umami?
- Uneyama et al. (2009) described umami as a mild but persisting aftertaste associated with salivation and furriness on the tongue that stimulates the throat, the roof and the back of the mouth.
- Rolls (2009) stated umami itself isn't palatable, but it makes a large variety of foods palatable with a matching aroma, nonetheless. Shizuko Yamaguchi (1998) stated that relatively low levels of umami is considered pleasant.
- Yamaguchi & Takahashi (1984) indicated the optimum umami taste is dependent on the amount of salt, and vice versa.
- Roininen et al. (1996) demonstrated that soups with umami were rated highly in terms of pleasantness, taste saltiness and ideal saltiness of low-salt soups, compared to low-salt soups without umami that were rated less pleasant.
- Huynh et al. (2016) found fish sauce (as an umami source) reduced the desire for salt by 10-25% to flavour certain foods such as chicken broth, coconut curry, or tomato sauce while maintaining overall taste intensity.
- Some groups, such as the elderly, have impaired taste and smell due to age and medication, which lead to poor nutrition, increasing the risk of disease. Masic & Yeomans (2014) found umami not only stimulates appetite, but as well as result in satiety in groups with impaired taste and smell.
What foods are rich in umami flavour?
- Naturally occurring glutamate are primarily found in meats and vegetables. Inosine (IMP) is mainly found in meats, whereas guanosine (GMP) is mainly found in vegetables.
- Mouritsen et al. (2014) found mushrooms (e.g. dried shiitake), smoked or fermented fish and shellfish are abundant sources of umami flavour from guanylate, inosinate and adenylate, respectively.
- Agostoni et al. (2000) demonstrated the amino acids in breast milk contain umami flavours, which tend to be the human's first taste of umami. It is known breast milk contains glutamic acid, which takes up about 50% of the free amino acids.
Umami flavours are primarily found in foods rich in L-glutamate, IMP and GMP. They include:
-- Fish
-- Shellfish
-- Meat extracts
-- Cured meats
-- Mushrooms
-- Vegetables (e.g. Chinese cabbage, celery, ripe tomatoes, spinach, etc.)
-- Green tea
-- Hydrolysed vegetable protein
-- Femented and aged products involving bacterial or yeast cultures (e.g. cheeses, fish sauces, nutritional yeast, shrimp pastes, soy sauce, and yeast extracts such as Vegemite and Marmite).
Describe the umami taste receptors

- A majority of taste buds on the tongue and other parts of the mouth can perceive umami taste, regardless of their location.
- San Gabriel et al. (2005) discovered the taste receptors that perceive the taste of umami are the modified forms of mGluR4, mGluR1, and taste receptor type 1 (TAS1R1 + TAS1R3). All of these taste receptors are located in all regions of the tongue that present taste buds. In addition, Sasano et al. (2015) found those aforementioned receptors are also located in some regions of the duodenum.
- A review by Finger (2009) summarised the list of candidates for umami receptors, which are the heterodimer TAS1R1/TAS1R3, and truncated type 1 and 4 metabotropic glutamate receptors lacking most of the N-terminal extracellular domain (taste-mGluR4 and truncated-mGluR1) and brain-mGluR4.
- In 1957, Akira Kuninaka found mGluR1 and mGluR4 are specific to glutamate whereas TAS1R1 and TAS1R3 are responsible for the synergism. However, the specific role of each type of receptor in taste bud cells aren't well understood.
- Kinnamon (2012) found G protein-coupled receptors (GPCRs) with similar signalling molecules such as G proteins βγ, PLCB2 and PI3 that modulate release of calcium (Ca2+) ions from intracellular stores.
- Zhang et al. (2003) stated calcium ions activate a transient-receptor potential action channel called TRPM5, which results in depolarisation of the cell membrane and the consequent release of ATP and secretion of neurotransitters such as serotonin.
- Clapp et al. (2004) stated cells responding to umami taste stimuli doesn't contain any synapses, however ATP relays taste signals to gustatory nerves and subsequently to the brain where it interprets and identifies the taste quality via the gut-brain axis.
Other taste sensations and transmissions
1. Pungency / Spiciness
- Pungency is defined as the food's spiciness, hotness, or heat level, such as chili peppers. Colloquially, pungency refers to any strong, sharp smell or flavour.
- Scientists prefer the word "pungent" to describe the "hotness" or "spiciness" of food in order to eliminate the ambiguity that derive from the term "hot", which may refer to temperature, and "spicy", which may refer to spices.
- For example, a pumpkin can be both hot (out of the oven) and spicy (due to the spices it contains such as cinnamon, allspice, cloves, mace and nutmeg), but it is not pungent. On the other hand, pure capsaicin is described as pungent, but it isn't naturally perceived as hot nor contains spices.
- Piquancy is referred to foods with low pungency that stimulate the palate, such as curry and mustard. Piquant flavours and spices are less intense than chilli peppers, including the strong flavour of some tomatoes.
Which foods are pungent?
- Pungent foods include a range of chilli peppers, black peppers and other spices such as ginger and horseradish. Their level of pungency is measured by the Scoville scale, as defined by the amount of capsaicin they contain.
- Pungent foods are common in a range of cuisines, such as Mexican, Spanish, Southwest Chinese (Sichuan), Korean, Jamican, Indian, Filipino, Malaysian, Thai, Vietnamese, Ethiopian, etc.
Describe the mechanism of pungency
- The skin and mucous membranes senses pungency through a process called chemesthesis. Pungent molecules such as capsaicin, piperine, thiosulfinates, allyl isothiocyanate, and allicin trigger a burning or tingling sensation by stimulating the trigeminal nerve along with typical taste reception.
- Pungent molecules activate heat thermo- and chemosensitive TRP ion channels including TRPV1 and TRPA1 nociceptors, which triggers that painful and burning hot sensation.
- Tewksbury et al. (2008) suggests the pungency of chilly peppers may be an adaptive response to microbial pathogens.
2. Coolness
Foods trigger that fresh, minty sensation despite not being at low temperatures include peppermint and spearmint due to the anethol, ethanol, camphor and menthol activating cold trigeminal receptors.
3. Numbness
Found in both Chinese and Batak Toba cooking, the notion of 麻 (má or mati rasa) is a tingling numbness caused by spices such as Sichuan pepper. Sichuan cuisune and North Sumatran cuisune tend to combine ma or mati rasa with chili pepper to make 麻辣 málà, or "mati rasa" flavour, meaning "numbing-and-hot".
In northern Brazilian cuisune, a herb called jambu is added to dishes such as tacacá to produce a numb flavour.
4. Astringency
Some foods, such as unripe fruits, contain tannins or calcium oxalate that induce an astringent or puckering perception of the mucous membrane of the mouth. Peleg et al. (1999) described the astringent taste as "dry", "tart", "hard", "harsh" (particularly for wine), "rough", "rubbery" or "styptic".
5. Metallicness
Metallic flavours may be found in certain foods and drinks, as well as medicines or amalgam dental fillings. This metallic perception is due to galvanic reactions occurring the mouth. A 2019 article reported dental fillings contain dissimilar metals that may produce a small current in the mouth. Other molecules perceived to have a metallic taste include artificial sweeteners and blood.
A metallic taste in the mouth may be a sign of a number medical conditions, such as dysgeusia or parageusia, a side-effect of certain medications such as saquinavir and zonisamide, or a side effect of chemotherapy, as well as occupational hazards, such as the presence of pesticides.
6. Fat taste
- Laugerette et al. (2005) found a potential taste receptor called the CD36 receptor, which is a possible lipid taste receptor that binds to fat molecules (particularly long-chain fatty acids).
- Furthermore, Simons et al. (2011) discovered CD36 is localised to taste bud cells, particularly the circumvallate and foliate papillae.
Scientists have argued over whether humans can actually taste fats.
- Supporters claimed the human's ability to taste free fatty acids (FFAs) provides an evolutionary advantage.
- Mattes (2011) found fatty acids trigger certain processes that activate gustatory neurons similar to other main tastes, as well as evoke a physiological response to its presence.
- Pepino et al. (2012) discovered an increase in expression of CD36 receptor correlated with an increase in sensitivity to fat.
- Cartoni et al. (2010) discovered G protein-coupled receptors GPR120 and GPR40 are associated with the taste of two types of fatty acids (linoleic acid and oleic acid), and increased nerve response to oral fatty acids.
- Liu et al. (2011) suggested the monovalent cation channel TRPM5 may play a role in fat taste, however Mattes (2011) believed this channel is mainly involved in downstream processing of the taste instead of primary reception, as is the case with bitter, sweet, and savoury tastes. Moreover, he described how low levels of fatty acids enhance the flavour in a food, and how high levels of fatty acids would render the food inedible. Mattes thought the fatty acid taste may be "warning" the person that a certain food should not be eaten.
- Russell Keast (2015) found humans were able to distinguish the taste of fatty acids from other tastes, but occasionally overlapping with savoury flavours, which may be due to unfamiliarity of both tastes.
- The researchers claimed the "creaminess and viscosity" of fatty foods is primarily due to triglycerides, which is unrelated to the taste, since the actual taste fatty acids is considered unpleasant.
7. Heartiness
- Kokumi (コク味) is a Japanese term meaning "heartiness", "full flavour", or "rich" that describes how the combination of colecules in food enhance their characteristics.
- It is described as enhancing the other 5 main tastes by boosting and amplifying the other tastes, or "mouthfulness". Ueda et al. (1990) alluded to garlic as a common ingrident to enhance kokumi flavours.
- Hettiarachchy et al. (2010) found calcium-sensing receptors (CaSR) perceive kokumi compounds, which trigger an increase in the intracellular calcium ion levels in a subset of taste cells. Eto et al. (2012) noted this subset of CaSR-expressing taste cells function independently from the basic taste receptor cells.
- Eto et al. (2010) found a basal level calcium that corresponds to the physiological concentration is sufficient to active CaSR in order to produce the sensation of kokumi.
8. Calcium
- Michael Tordorf (2008) discovered a calcium receptor on the tongues of mice called the CaSR receptor, which is also found in the brain, gastrointestinal tract, and kidneys.
- A 2014 Scientific American article described the calcium content in chalk as having a distinctive taste.
- However, it is currently unknown whether humans can actually perceive the taste of calcium.
9. Temperature
- Food served hot or warm can emphasise some flavours and decrease other flavours by modifying the density and phase equilibrium of the food molecules. In some cultures, food and drink served cold are regarded as repugnant, thus are traditionally served hot, and vice versa.
- For instance, most alcoholic beverages are traditionally served at served at room temperature or chilled to varying degrees, whereas most soups are usually served hot.
Other taste phenomena
a. Supertaster
- When a person's sense of taste has a higher intensity than the average person's sense of taste, they are described as a supertaster.
- The term was coined by experimental psychologist Linda Bartoshuk, who discovered a number of individuals demonstrating an elevated taste response.
- She noted this elevated taste response is not due to response bias or a scaling artifact but rather due to an anatomical or biological cause.
- In 1931, a DuPont chemist named Arthur L. Fox discovered a number of individuals perceived phenylthiocarbamide (PTC) as bitter, whereas others perceived it to be tasteless. At the 1931 American Association for the Advancement of Science meeting, Fox collaborated with a geneticist named Albert F. Blakeslee to have attendees taste PTC. The results were 65% of them perceived it as bitter, 28% perceived it as tasteless, and 6% perceived it as other tastes.
- In the 1960s, Roland Fischer was the first person to correlate the ability to taste PTC, and the related substance propylthiouracil (PROP), to food preference, diets, and calorie intake.
- Researchers estimated 25% of the population are non-tasters, 50% are medium tasters, and 25% are supertasters.
What are the causes of supertaste?
- As of today, the exact cause of supertaste in humans is not well understood. Bartoshuk et al. (1994) discovered links between supertasters and the TAS2R38 gene, the ability to taste PROP and PTC, and an increased number of fungiform papillae.
- The mechanisms by which environmental factors affect sensitive taste, as well as possible evolutionary advantages to increased taste sensitivity, are currently unknown.
- It is theorised that an elevated response to bitter flavours would help avoid potentially toxic plant alkaloids, but limit consumption of non-toxic palatable foods.
- Researchers suggested a link between the bitter-taste-receptor TAS2R38 and the ability to taste PROP and PTC, although a casual relationship with elevated taste sensitivity hasn't been proven.
- Furthermore, researchers claimed an correlation between the T2R38 genotype and a preference for sweet flavours in children, avoidance of alcoholic beverages and cigarette smoking, and increased prevalence of colon cancer (due to inadequate vegetable consumption).
b. Aftertaste
- If you perceive an intense taste of a food or beverage immediately after it leaves your mouth, it is an aftertaste.
- Neely & Borg (1999) described the features of a food's aftertaste are its duration, intensity and quality. Quality describes the food's actual taste, intensity indicates the taste's magnitude and duration is defined as the length of time of a food's aftertaste sensation. For example, taste quality tend to be described as bitter, salty, sour, sweet umami, or no taste.
- Studies employed a number of scales such as the Borg Categoy Ratio Scale to assess the intensities of foods. Scales contain categories that range from either 0 or 1 - 10 or 10+ to describe the food's taste intensity. Logically, 0 or 1 indicates weaker taste intensities, whereas scores of 6 or higher indicates moderate or stronger taste intensities. If the moderate or strong taste intensities linger after the food leaves the mouth, it is regarded as an aftertaste sensation.
- A food's temporal profile includes the time of onset for the initial taste sensation during food consumption, and subsequent recording of the time at which the taste sensation disappears. The difference between the 2 time values results in the total time of taste reception, which then corresponds with intensity intensity assessments over the same time interval and a representation of the food's taste intensity over time is yielded.
- Measuring the onset of taste perception from the point after which the food leaves the mouth determines how long the aftertaste perception lasted.
Describe the dynamics of aftertaste
- The molecular mechanisms responsible for aftertaste are postulated to be associated with either the continued or delayed activation of taste receptors and signalling pathways in the mouth that play a role in taste processing.
- When food particles bind to taste receptors on taste cells situated on the tongue and the roof of the mouth. Temporal and spatial factors such as the time of taste receptor activation or the particular taste receptors being activated (e.g. bitter, sweet, salty, sour, umami) can influence those chemical-receptor interactions.
- The chorda tympani (cranial nerve VII), the glossopharyngeal nerve (cranial nerve IX), and the vagus nerve (cranial nerve X) then transmit information from the taste receptors to the brain for cortical processing.
- Peri et al. (2008) suggested the combination of both receptor-dependent and receptor-independent processes are involved in the signal transduction mechanisms of foods with aftertastes. The receptor-dependent process is the same as aforementioned above, however the receptor-independent process involves the diffusion of bitter, amphiphilic chemicals such as quinine across the taste receptor cell membranes.
- When food molecules enter the taste receptor cell, they activate intracellular G-proteins and other signalling proteins that are involved in signal transduction pathways linked to the brain.
- It's known that bitter compounds activate both the taste receptors on the cell surface, as well as the signalling pathway proteins in the intracellular space. Intracellular signalling runs slower than taste cell receptor activation possibly due to extra time required for the bitter compounds to diffuse across the cell membrane and interact with intracellular proteins.
- Naim et al. (2002) suggested this slowed activation of intracellular signalling proteins in response to the bitter compounds, in addition to the extracellular receptor signalling may be associated with the persisting aftertaste linked to bitter foods.
- It's thought the combination of both processes results in an overall longer response of the taste receptor cells to bitter compounds, and aftertaste perception subsequently occurs.
Taste Processing in the cerebral cortex
- The main taste perception regions in the cerebral cortex are located in the insula and areas in the somatosensory cortex, well as the nucleus of the solitary tract in the brainstem. In the case of aftertaste, not much is known about the cortical processing associated with its perception.
- James et al. (2009) studied the temporal taste profile of an artificial sweetener called aspartame in humans. They found the insula was activated for longer periods than other sensory processing brain regions after analysing the aftertaste profile of aspartame.
- Prior to aspartame consumption, the amygdala, basal ganglia, somatosensory cortex, and thalamus were all activated. After consumption, only the insula remained actiavted and other brain regions were observed to be less activated.
- This indicated that the insula plays a major role in aftertaste sensation since it was still activated after the aspartame solution left the mouth. This supports the theory that the insula functions as a central taste processing area.
Which foods have distinct aftertastes?
- Low-calorie artificial sweeteners such as acesulfame-K and saccharin have a bitter aftertaste. Slack et al. (2010) used GIV3727 (4-(2,2,3-trimethylcyclopentyl) butanoic acid) to block bitter taste receptors from binding to the saccharin and acesulfame-K solutions. As a result, this significantly decreased the taste intensity rating compared to normal solutions of artificial sweeteners.
- Wine tasters determine the wine's aftertaste when evaluating its quality. The wine's aftertaste can be sweet, smooth, bitter, short, persistent or non-existent. Wines adjudged to be high quality tend to have persistent aftertastes combined with pleasant aromas.
c. Acquired taste
- An acquired taste is defined as appreciating a taste that is unlikely to be appreciated by a person who lacks exposure to it. The opposite of acquired taste is innate taste.
- If one experiences difficulty enjoying their food or drink, it may be due to a strong odour, taste, mouthfeel, appearance, or association with disgust.
- Strong odour: Blue cheese, durian, surströmming, stinky tofu
- Intense taste: Alcohol, coffee, vegemite / marmite, bitter tea, liquorice, dark chocolate, haggis, malt bread, Asian pickles, etc.)
- Mouthfeel: Sashimi and sushi containing raw seafood
- Association with disgust: Insects, organ meat such as ox tongue.
- The mechanism of taste acquisition involves developmental maturation, genetics (of both taste sensitivity and personality), biochemical reward characteristics of food, and family tree.
- Infants are born with a strong preference for sweet foods and a strong rejection of sour and bitter foods, and then develop a preference for salty foods about 4 months later.
- As infants eat more and more solid foods, they generally accept various foods, however toddlers and young children can sometimes experience neophobia (fear of novelty) towards food. Nevertheless, older children, adults and elderly people are less neophobic and become adventurous with their food by widening their food palette.
- Ullrich et al. (2004) found supertasters are highly sensitive to bitter, pungent and spicy flavours, which means they tend to avoid them and prefer to eat milder, plainer foods. However, numerous supertasters demonstrate high food adventurousness in order to seek these intense flavours.
- Smit & Blackburn (2005) found supertasters preferred the drinks containing caffeine and theobromine (active compounds in chocolate) than a placebo drink containing the same flavours. This suggests combinations of natural compounds in foods offer both flavour and nutritional benefits to the body and mind, which result in an acquired taste.
- Bovens (1992) stated intentionally altering one's acquired taste preferences from inauthentic acquired tastes motivated by conformity or status is challenging and difficult to achieve. This may make it difficult to distinguish authentic from legitimate acquired tastes.
Describe the neural pathways of taste
- 1/3rd of the tongue include the circumvallate papillae is innervated by the glossopharyngeal nerve, while the other 2/3rds of the tongue and the cheek is innervated by the facial nerve via the chorda tympani.
- The pterygopalatine ganglia are located on each of the side of the soft palate, where the greater petrosal, lesser palatine and zygomatic nerves all synapse.
- The greater petrosal nerve transmits taste signals from the soft palate to the facial nerve.
- The lesser palatine nerve transmits signals to the nasal cavity, which explains why spicy foods result in nasal drip.
- The zygomatic nerve transmits signals to the lacrimal nerve that activate the lacrimal gland, which explains why spicy foods results in tears.
- In addition, both the lesser palatine and zygomatic nerves are maxillary nerves, meaning they originate from the trigeminal nerve.
- The special visceral afferents of the vagus nerve transmit taste information from the epiglottal area of the tongue to the brain.
- The lingual nerve is interconnected with the chorda tympani, in which provides all sensory information from the anterior 2/3 of the tongue.
- This sensory information is processed separately in the rostal lateral subdivision of the nucleus of the solitary tract (NST).
- It's known the NST receives input from the amygdala (a regulator of oculomotor nuclei output), bed nuclei of stria terminalis, hypothalamus, and prefrontal cortex. King & Travers (1999) described the NST as the topographical map that processes gustatory and sensory (temp, texture, etc.) information.
- The reticular formation (including Raphe nuclei) is activated to release serotonin during and after a meal to suppress appetite. In addition, salivary nuclei are activated to reduce the secretion of saliva.
- Hypoglassal and thalamic nuclei play in a role in oral movements during eating.
- The hypothalamus releases hormones to regulate hunger and the digestive system.
- Taste stimuli triggers the Edinger-Westphal nucleus, which results in dilation and constriction of the pupils.
- The insula cortex plays an important role in swallowing and gastric motility.
6. What are teeth?
- A tooth (plural: teeth) is a sturdy, calcified structure situated in the jaws (or mouths) of vertebrates and its main function is to disintegrate food. The roots of teeth are embedded and surrounded by gums.
- Some animals, such as carnivores and omnivores, use their teeth to catch or injure prey, rip apart food, for defensive purposes, to terrify other animals, or to carry prey or their offspring.
- Note that teeth aren't composed of bone, but rather a multitude of tissues of varying density and sturdiness that derive from the ectoderm. The general structure of teeth is much the same across the vertebrates, in spite of the fact there is variation in their form and position.
- The teeth of mammals, as well as some fish and crocodiles, contain deep roots. In a majority of teleost fish, the teeth are connected to the outer surface of the bone, whereas the teeth in lizards are connected to the inner surface of the jaw by one side.
- The teeth in cartilaginous fish, such as sharks, are connected by strong ligaments to the cartilage hoops that form the jaw.
- Monophyodonts develop only 1 set of teeth, while diphydonts (e.g. humans) develop an early set of deciduous teeth and then a later set of permanent ("adult") teeth. Polyphyodonts, such as sharks, elephants, kangaroos, and manatees, develop numerous sets of teeth in order to replace eroded teeth.
- The more rodent gnaw, their incisors develop and erode, which allows them to maintain a relatively consistent length. Tummers & Thesleff (2003) found some rodents, such as guinea pigs and voles (but not mice), as well as lagomorpha (hares, pikas and rabbits), have continuously developed molars as well as incisors.
- The teeth of mammals are typically connected to the jaw, whereas the teeth of numerous reptiles and fish are typically connected to the palate or to the floor of the mouth. In addition, the teeth in reptiles and fish are organised in several rows inside those on the jaws proper.
- Romer & Parsons (1977) suggested teeth first evolved in sharks, since they aren't found in more primitive jawless fish. Although lampreys contain tooth-like structures on their tongues, they are composed of keratin, rather than of dentine or enamel, meaning they aren't associated with true teeth. The structure of dermal denticles found in sharks are virtually identical to true teeth, which indicates they have a shared evolutionary origin.
- Matt Kaplan (2013) found teeth-like structures composed of dentine and enamel in late conodonts, which implies they have evolved independently of later vertebrates' teeth.
- Amphibians usually contain a number of small teeth, or no teeth at all, because they tend to feed on soft foods.
- Reptiles have conical teeth that vary in appearance between species, such as snakes with fangs that inject venom.
- The teeth of mammals (e.g. humans) are organised in the pattern of incisors, canines, premolars and molars. In general, incisors slice the food, canines shred the food, molars and premolars macerate the food. The roots of mammals' teeth are lodged in the maxilla (upper jaw) or the mandible (lower jaw) and are enveloped by gums.
I will delve into the details of teeth and dental anatomy in another post.
7. Epiglottis
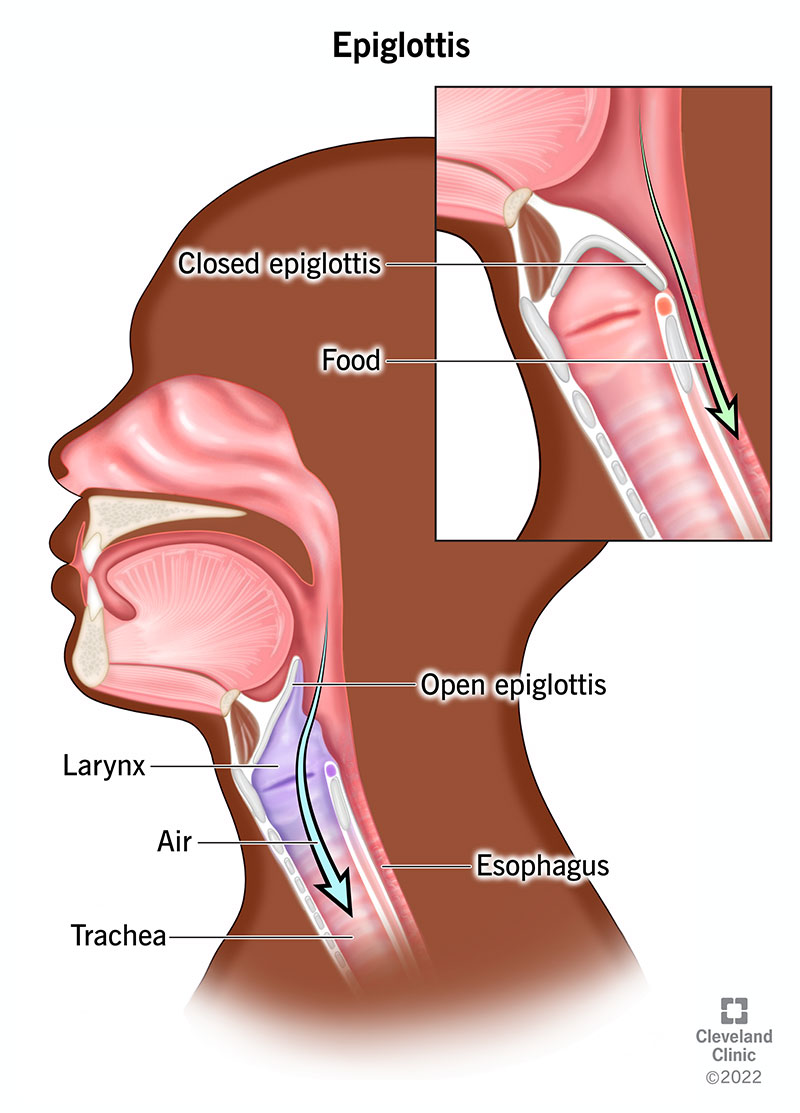
- The epiglottis is a small flap in the throat that stops food and water from entering the trachea and the lungs.
- It opens during the breathing process, which permits the flow of air into the larynx and lungs. On the other hand, it closes to avoid aspiration of food into the lungs, which propels the swallowed food or liquids down the oesophagus to the stomach instead.
- The epiglottis was discovered as early as Aristotle, however its function was first described by Vesalius in 1543.
- Its name is derived from being above (Ancient Greek: ἐπί, romanized: epi-) the glottis (Ancient Greek: γλωττίς, romanized: glottis, lit. 'tongue').
Describe the structure of the epiglottis
- The epiglottis is a leaf-shaped flap located at the entrance of the larynx. It has a free upper segment that sits behind the tongue, and a lower stalk that originates from the back surface of the thyroid cartilage, joined by a thyroepiglottic ligament. The stalk is joined to the arytenoid cartilages at the walls of the larynx by folds at the sides.
- The epiglottis originates at the entrance of the larynx, and joins to the hyoid bone. Then it extends upwards and backwards behind the tongue. The space betwen the epiglottis and the tongue is known as the vallecula.
Development
- The epiglottis originates from the 4th pharyngeal arch, which appears later than the other cartilage of the pharynx, around the 5th month of development.
- In infants, the position of the epiglottis makes contact with the soft palate, which shifts downwards with age.
Microanatomy
- The epiglottis has 2 surfaces: an anterior surface facing forwards, and a posterior surface facing the larynx.
- The anterior surface is coated by layers of stratified squamous epithelium, whereas the posterior surface is coated by a layer of columnar epithelium with cilia. In addition, the posterior surface contains goblet cells that produce mucus. The intermediate zone between these surfaces consist of cells that change shape.
- The body of the epiglottis contains elastic cartilage, and its surface contains taste buds.
Describe the functions of the epiglottis
- During the breathing process, the epiglottis typically orients upwards with its underside serving as part of the pharynx.
- During the swallowing process, the epiglottis bends backwards, which turns over the entrance to the trachea to stop from entering into it. However, the mechanism behind its backward movement is not well understood.
- It is theorised that the hyoid bone and the larynx shift upwards and forwards, which raises passive pressure from the back of the tongue. This is due to contraction of the aryepiglottic, laryngeal and thyroarytenoid muscles, as well as the passive weight of the food exerting downwards force. This allows the epiglottis to block passage to the trachea, which forces the food to travel down the oesophagus.
- The epiglottis is usually not required to produce sounds in numerous languages. However, in some languages such as Amis, Archi, Dahalo, Haida and Ingush, the epiglottis functions as an articulator to make the pharyngeal consonants and in the vowel /a/. This consonantal sound is known as the epiglottal or pharyngeal plosive.
8. Pharynx
- The pharynx is a section of the throat behind the mouth and the nasal cavity, and above the oesophagus and trachea. The word pharynx comes from the Greek φάρυγξ phárynx, meaning "throat". Its plural form is pharynges /fəˈrɪndʒiːz/ or pharynxes /ˈfærɪŋksəz/, and its adjective form is pharyngeal (/ˌfærɪnˈdʒiːəl/ or /fəˈrɪndʒiəl/).
Describe the structure of the pharynx
i. Nasopharynx
- The upper section of the pharynx is called the nasopharynx, which stretches from the base of the skull to the upper surface of the soft palate. It includes the space between the internal nares and the soft palate, situated above the oral cavity.
- Known as the pharyngeal tonsils, the adenoids are lymphoid tissue structures in the posterior wall. It is lined by respiratory epithelium that is ciliated, columnar and pseudostratified.
- The link between the middle ear and the nasopharynx at the pharyngeal opening is the auditory tube. By opening and closing, the auditory tubes equalises the barometric pressure in the middle ear with that of the atmosphere.
- The anterior part of the nasopharynx communicates through the choanae with the nasal cavities. The pharyngeal opening of the auditory tube is located on its lateral wall, which has a triangular shape and is bounded behind the torus tubarius or cushion. This is caused by the medial end of the cartilage of the tube that lifts the mucous membrane.
2 folds emerge from the cartilaginous opening:
- Salpingopharyngeal fold = A vertical fold of mucous membrane that stretches from the interior aspect of the torus. It contains the salpingopharyngeus muscle.
- Salpingopalatine fold = Located in the front of the salpingopharyngeal fold, this smaller fold stretches from the superior aspect of the torus to the palate. It contains the levator veli palatini muscle. The tensor veli palatini is located laterally to the levator and doesn't play a role in the fold's movements.
ii. Oropharynx
- The orophraynx is located behind the oral cavity, stretching from the uvula to the hyoid bone. It opens anteriorly into the mouth via the isthmus faucism.
- A structure inside its lateral wall, located between the palatoglossal arch and the palatopharyngeal arch, is the palatine tonsil. The oropharynx is lined by non-keratinised squamous stratified epithelium.
- The anterior wall contains the base of the tongue and the epiglottic vallecula, while the lateral wall consists of the tonsil, tonsillar fossa, and the tonsillar (faucial) pillars. The superior wall contains the inferior surface of the soft palate and the uvula.
- Since both food and air pass through the pharynx, the epiglottis folds over and blocks the glottis whenever food is swallowed to avoid aspiration.
- The HACEK organisms (Haemophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella) are part of the oropharyngeal flora, which undergo gradual development. They prefer an environment that is rich in carbon dioxide, and share an enhanced capacity to create endocardial infectious, esp. in young children.
iii. Laryngopharynx
- Known as the hypopharynx, the laryngopharynx is the caudal section of the pharynx, which links to the oesophagus. It is located inferior to the epiglottis and stretches to the junction of the respiratory (laryngeal) and digestive (oesophageal) pathways. Its wall is lined with stratified squamous epithelium.
- At that point, the laryngopharynx is continuous with the oesophagus posteriorly, meaning food and fluids flows down to the stomach, whereas air flows through the larynx anteriorly.
- The superior boundary of the laryngopharynx is in line with the hyoid bone, which corresponds to a section between the 4th and 6th cervical vertebrae.
- It contains 3 major locations: the postcricoid area, the posterior pharyngeal wall and the pyriform sinus.
- The lingual artery, the ascending pharyngeal artery and the superior thyroid artery provides vascular supply to the laryngopharynx.
- Both the vagus and glossopharyngeal nerves provide nerve supply to the laryngopharynx.
9. Oesophagus
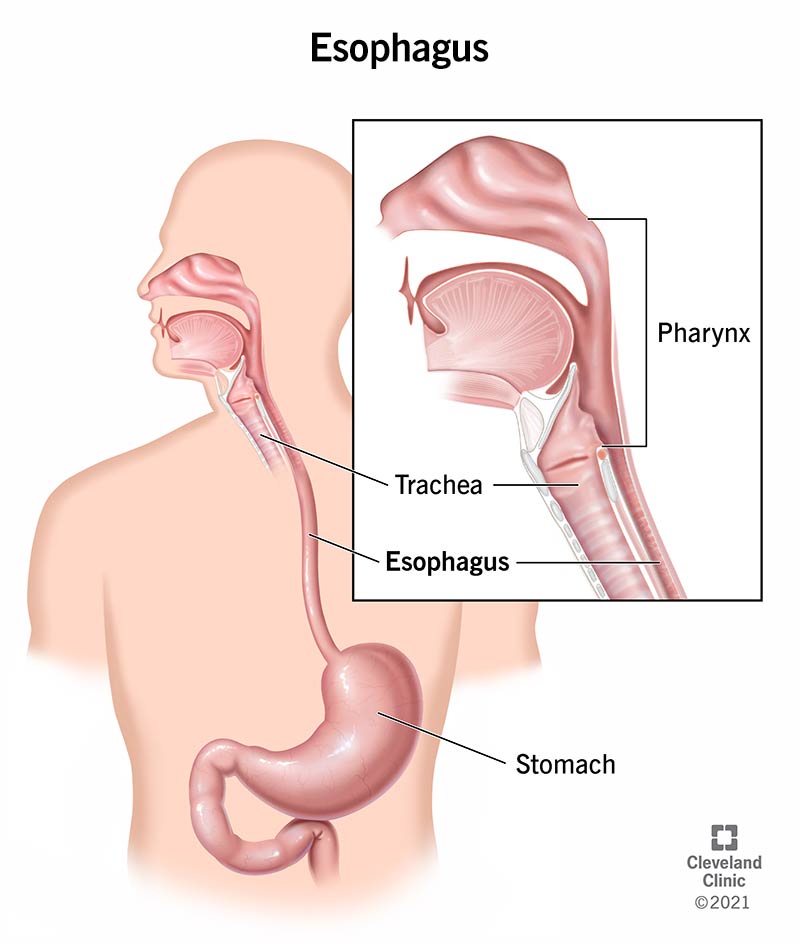
- Colloquially known as the food pipe, food tube, or gullet, the oesophagus (British English) or esophagus (American English) is an organ in vertebrates that food and fluids travels through from the pharynx to the stomach.
- The world oesophagus derives from the Greek: οἰσοφάγος (oisophagos) meaning gullet. It is a combination of 2 words; eosin (to carry) and phagos (to eat).
- The earliest use of the word oesophagus has been documented since at least the time when Hippocrates lived, who said "the oesophagus ... receives the greatest amount of what we consume".
- Roman naturalist Pliny the Elder (AD23–AD79) documented the existence of the oesophagus in other animals and its continuity with the stomach. The observation of the peristaltic contractions of the oesophagus was documented since at least when Galen existed.
- In 1871, Theodore Billroth performed the first surgery attempt on the oesophagus in the neck region of dogs. In 1877, Czerny performed similar surgery in human patients.
- In 1908, Voeckler performed an operation to extract the oesophagus, and in 1933 the first successful surgical removal of sections of the lower oesophagus was performed in a patient with oesophageal cancer.
- In 1955, Rudolph Nissen performed a fundoplication, which involves wrapping the stomach around the lower oesophageal sphincter to trigger its function and control reflux.
Gene and protein expression
- Uhlén et al. (2015) estimated approximately 20,000 protein-coding genes are expressed in human cells and roughly 70% of these genes are expressed in the oesophagus. About 250 of thes genes are specifically expressed in the oesophagus with less than 50 genes being highly specific.
- The corresponding oesophagus-specific proteins are involved in squamous differentiation such as keratins KRT13, KRT4 and KRT6C. Mucins such as MUC21 and MUC22 lubricate the inner surface of the oesophagus.
- Edqvist et al. (2014) found a number of oesophageal genes with increased expression are shared with the skin and other organs that are composed of squamous epithelia.
Development of the oesophagus
- In early embryogenesis, the oesophagus develops from the endodermal primitive gut tube. During week 2 of embryological development, the growing embryo starts to envelop sections of the sac, which set up the foundation for the adult gastrointestinal tract.
- The sac is surrounded by a network of vitelline arteries, which gradually consolidate into the 3 main arteries that supply the developing gastrointestinal tract (GIT): the celiac, inferior mesenteric and superior mesenteric arteries. Schoenwolf (2009) used these regions supplied by these arteries to define the midgut, hindgut and foregut.
- The surrounded sac develops into the primitive gut, which start to differentiate into the main organs of the gastrointestinal tract, such as oesophagus, the stomach, and the intestines. Furthermore, the oesophagus develops as part of the foregut.
Describe the structure of the oesophagus
 | |
| This is an illustration of the oesophagus's position and relation in the cervical region and in the posterior mediastinum viewed from behind. |
- The oesophagus starts from the back of the mouth, stretching downward through the back part of the mediastinum, through the diaphragm, before connecting to the stomach. On average, the oesophagus is roughly 25 cm (10 in) in length in an adult human.
- In humans, the oesophagus typically begins around C6 level (6th cervical vertebra) behind the cricoid cartilage of the trachea, then enters the diaphragm at around T10 level (10th thoracic vertebra), and ends at the cardia of the stomach at around T11 level (11th thoracic vertebra).
- The upper sections of the oesophagus and the upper oesophageal sphincter receive blood from the inferior thyroid artery.
- Sections of the oesophagus in the thorax receive blood supply from the bronchial arteries as well as branches directly from the thoracic aorta.
- The lower sections of the oesophagus and the lower oesophageal sphincter receive blood from the left gastric artery and the left inferior phrenic artery.
Blood drainage:
- The upper and middle sections of the oesophagus drain into the azygos and hemiazygos veins, whereas the lower section drains into the left gastric vein.
- All of these veins except for the left gastric vein drain into the superior vena cava. In fact, the left gastric vein is a branch of the portal vein.
Lymph drainage:
- The upper 3rd of the oesophagus drains into the deep cervical lymph nodes.
- The middle 3rd of the oesophagus drains into the superior and posterior mediastinal lymph nodes.
- The lower 3rd of the oesophagus drains into the gastric and coeliac lymph nodes.

i. Position
- The upper oesophagus is located at the back of the mediastinum behind the trachea, adjacent to the tracheoesophageal stripe, and in front of the erector spinae muscles and the vertebral column.
- The lower oesophagus is located behind the heart and bends in front of the thoracic aorta. From the bifurcation of the trachea downwards, the oesophagus extends behind the right pulmonary artery, left main bronchus, and left atrium. Then it passes through the diaphragm.
- The thoracic duct goes behind the oesophagus, then bending from behind the oesophagus on the right in the lower section, to behind the oesophagus on the left in the upper oesophagus.
- In addition, the oesophagus is located in front of sections of the hemiazygos veins and the intercostal veins on the right hand side. The vagus nerve splits and envelops the oesophagus in a plexus.
ii. Constrictions
There are 4 points of constriction in the oesophagus:
- 1. At the beginning of the oesophagus, where the laryngopharynx links to the oesophagus, behind the cricoid cartilage.
- 2. Where it is intersected by the aortic arch on the front in the superior mediastinum.
- 3. Where the oesophagus is compressed by the left main bronchus in the posterior mediastinum
- 4. The oesophagus hiatus - where it passes through the diaphragm in the posterior mediastinum
When a corrosive substance, or a solid object is swallowed, it may lodge or damage 1 of the 4 constructions.
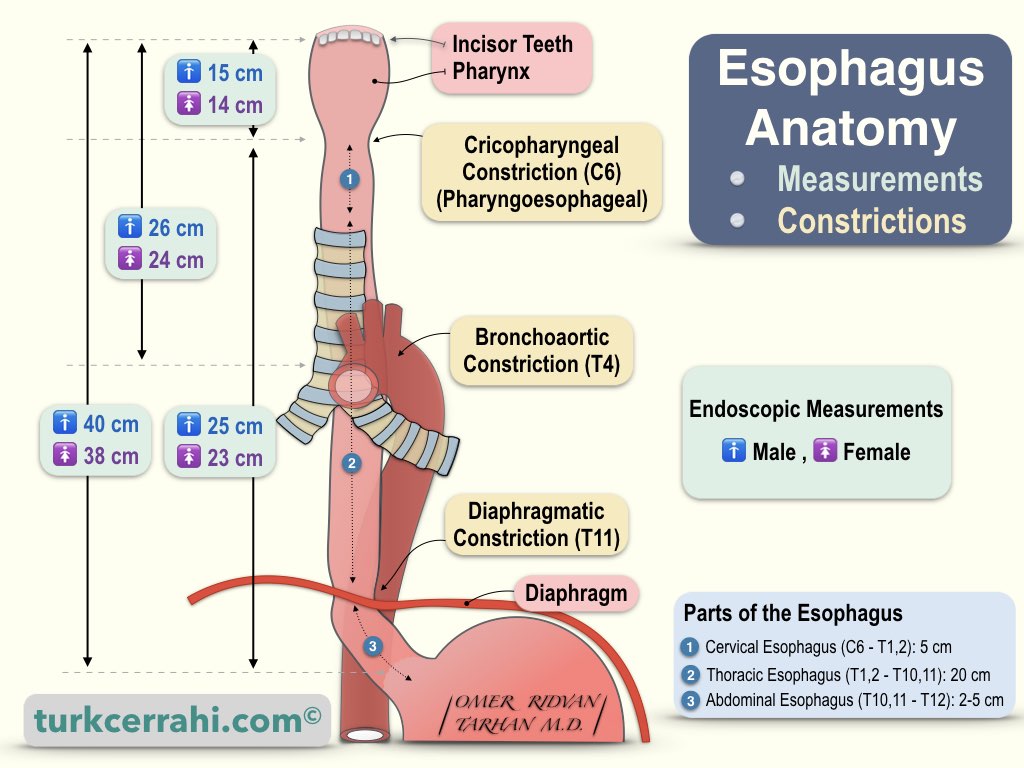
iii. Sphincters
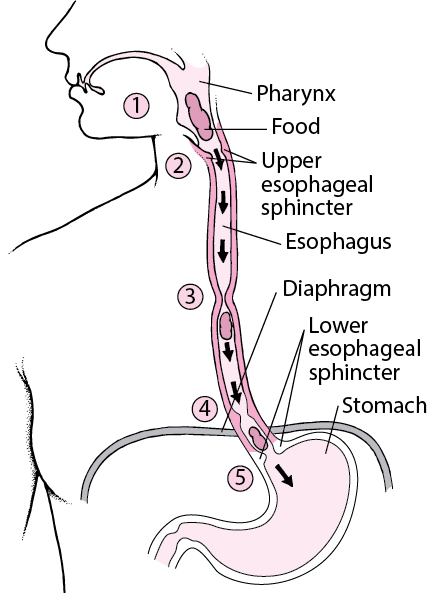
- The oesophagus is enclosed by 2 muscular rings at the top and bottom, called respectively as the upper oesophageal sphincter and the lower oesophageal sphincter. The main function of the sphincters is to close the oesophagus when no food or fluid is being swallowed.
- The upper oesophageal sphincter is an anatomical sphincter that is created by the lower section of the inferior pharyngeal constrictor, also known as the cricopharyngeal sphincter due to its anterior relation with cricoid cartilage of the larynx. It surrounds the upper section of the oesophagus, and consists of skeletal muscle but is under involuntary control. The swallowing reflex can stimulate the opening of the upper oesophageal sphincter. Mu et a. (2007) described the primary muscle of the upper oesophageal sphincter is the cricopharyngeal portion of the inferior pharyngeal constrictor.
- In contrast, the lower oesophageal sphincter is not an anatomical but instead a functional sphincter, which means it serves as a sphincter without the distinct thickening like other sphincters. Also known as the gastroesophageal sphincter, the lower oesophageal sphincter surrounds the lower section of the oesophagus at the junction between the oesophagus and the stomach. Alternatively, it is also called the cardiac sphincter or cardioesophageal sphincter, which is named from the cardia, the adjacent section of the stomach.
- If the gastroesophageal sphincter experiences dysfunction, it leads to gastroesophageal reflux, which causes heartburn.
iv. Nerve supply
- The oesophagus is innervated by the vagus nerve, as well as the cervical and thoracic sympathetic trunk. The vagus nerve has a parasympathetic path that innervates the muscles of the oesophagus and stimulates glandular contraction.
- Neurons originating from the nucleus ambiguus innervate the upper oesophageal sphincter and the upper striated muscle. On the other hand, neurons originating from the dorsal motor nucleus innervate the lower oesophageal sphincter and the smooth muscle.
- The sympathetic trunk triggers peristalsis and glandular activity, which results in contraction of the sphincters. Furthermore, sympathetic activity can result in muscle wall relaxation and blood vessel constriction.
- Both the vagus nerve and the sympathetic trunk provide nerve sensation along the length of the oesophagus, with gross sensation perceived by the vagus nerve and pain sensation perceived by the sympathetic trunk.
v. Gastroesophageal junction
- The gastroesophageal junction is located between the lower end of the oesophagus and the stomach. The main colour of the oesophageal mucosa is pink, whereas the main colour of the gastric mucosa is deep red. Gore & Levine (2010) described the mucosal transition as an irregular zig-zag line, which is referred to as the z-line.
- An histological examination of the gastroesophageal junction by Moore & Agur (2002) demonstrates an immediate transiton between the straified squamous epithelium of the oesophagus and the simple columnar epithelium of the stomach.
- The cardia of the stomach is situated abruptly distal to the z-line and the z-line corresponds with the upper limit of the gastric folds of the cardia. In the case of the mucosa's anatomy being deformed in Barrett's oesophagus, the actual gastroesophageal junction can be idenfied by the upper limit of the gastric folds rather than the mucosal transition.
- The functional location of the lower oesophageal sphincter is roughly 3 cm (1+1⁄4 in) below the z-line.
vi. Microanatomy
- The human oesophagus contains a mucous membrane that consists of a stratified squamous epithelium without keratin, a smooth lamina propria, and a muscularis mucosae.
- The epithelium of the oesophagus has a relatively quick turnover and facilitates protection against the abrasive effects of food. If a layer of keratin is present in the epithelium, it indicates the animal's diet is relatively coarse.
- 2 kinds of glands exist in the oesophagus: the mucus-secreting oesophageal glands located in the submucosa, and the oesophageal cardiac glands located in the lamina propria and the terminal region of the oesophagus.
- The mucus secreted from the glands provide a protective layer to the epithelial lining. The submucosa contains the submucosal plexus, which is a network of neurons that forms part of the enteric nervous system.
- The top third of the oesophagus consists of striated muscle, the lower third consists of smooth muscle, and the middle third consists a combination of both.
- Muscle in the oesophagus is arranged in 2 layers: (1) muscle fibres running longitudinal to the oesophagus, and (2) the muscle fibres surrounding the oesophagus.
- Each muscle layer is separated by the myenteric plexus, which is a meshed network of nerve fibres that plays important roles in mucus secretion and in peristalsis of the smooth muscle.
- A majority of the outermost layer of the oesophagus is the adventitia, whereas the abdominal region is surrounded by serosa.
10. Diaphragm
- The muscular diaphragm is located between the thoracic cavity and the abdominal cavity where a majority of the digestive organs are located.
- The suspensory muscle joins the ascending the duodeum to the diaphragm, which is believed to provide a wider angle to the duodenojejunal flexure for the smoother passage of digesting food.
- Moreover, the diaphragm joins to, and anchors the liver at its bare area. The oesophagus enters the abdomen through an orifice in the diaphragm at T10 level.
- I will delve into the diaphragm in detail in another post.
B. Gastric phase
This stage occurs in the stomach, where the food bolus is further disintegrated by combining with gastric acid until it passes into the duodenum, the first section of the small intestine.
11. Stomach
Describe the structure of the stomach
- The stomach is a hollow organ in the gastrointestinal tract of animals, including some invertebrates. The word stomach comes from the Greek stomachos (στόμαχος), from stoma (στόμα) 'mouth'. The prefixes gastro- and gastric (meaning 'related to the stomach') are both derived from Greek gaster (γαστήρ) meaning 'belly'.
- In humans, the stomach is located between the oesophagus and the duodenum, in the left upper quadrant of the abdominal cavity. The top part of the stomach leans against the diaphragm and the pancreas is lies posterior to the stomach.
- A large double fold of visceral peritoneum suspended from the greater curvature of the stomach is called the greater omentum.
- The stomach contains 2 sphincters: the lower oesophageal sphincter (in the cardiac region, at the junction of the oesophagus and stomach), and the pyloric sphincter (at the junction of the stomach with the duodenum).
- The stomach is surrounded by sympathetic and parasympathetic plexuses, which modulate both the secretory activity of the stomach and the motor activity of the stomach muscles.
- Since it's a distensible organ, the stomach expands to contain about 1 litre of food. The stomach of newborn human babies can retain about 30 mm, whereas the stomach of an adult human can hold between 2 and 4 litres.
i. Sections
The human stomach consists of 4 sections.
a. Cardia = Contents from the oesophagus empty here. This is the region that follows the "z-line" of the gastroesophageal junction, the point at which the epithelium alters from stratified squamous to columnar. The lower oesophageal sphincter is located adjacent to the cardia of the stomach. Lenglinger et al. (2012) found that the cardia is not an anatomically distinct region of the stomach but rather a region of the oesophageal lining damaged by reflux.
b. Fundus = Latin for 'bottom', it forms the upper curved section.
c. Body = Central area of the stomach
d. Pylorus = Greek for 'gatekeeper', it is the lower section of the section that empties contents into the duodenum.
ii. Blood supply
- The left gastric artery superiorly and the right gastric artery inferiorly supplies the lesser curvature of the human stomach, as well as the cardiac region.
- The right gastroepiploic artery inferiorly and the left gastroepiploic artery superiorly supplies the greater curvature of the stomach.
- Branching from the splenic artery are the short gastric arteries, which supply the fundus region of the stomach, as well as the upper section of the greater curvature.
iii. Microanatomy
- Stomach wall
- The human stomach wall is composed of mucosa, submucosa, muscularis externa, subserosa and serosa.
- The inner region of the stomach's lining, known as the gastric mucosa, contains an outer layer of column-shaped cells called a lamina propria, and a thin layer of smooth muscle called the muscularis mucosa.
- The submucosa is located beneath the mucosa, which contains fibrous connective tissue. Meissner's plexus is located in the submucosa, interior to the oblique muscle layer.
- The muscularis externa lies outside the submucosa, which contains 3 layers of muscular fibres. They are the called the inner oblique, middle circular, and outer longitudinal layers. Since the stomach contains the thickest muscularis layer, maximum peristalsis typically occurs in this region.
- A 2021 study found the inner oblique layer is not found in any other region of the gastrointestinal tract outside of the stomach.
- The inner oblique layer = This layer plays a role in producing the stomach movements that churns and disintegrates the food. The skin cells of the antrum are thicker in its walls and produces more powerful contractions than the fundus.
- The middle circular layer = The pylorus is surrounded by a thick circular muscular wall, which is typically tonically constricted. This creates a functional pyloric sphincter, which controls the flow of chyme into the duodenum. This layer is concentric to the longitudinal axis of the stomach.
- Also known as Auerbach's plexus, the myenteric plexus is located between the outer longitudinal and the middle circular layer. It plays a role in innervating both players, which results in peristalsis and mixing.
- The outer longitudinal layer serves to shift the bolus towards the pylorus of the stomach through muscular shortening.
- Stomach glands
https://en.wikipedia.org/wiki/Gastric_glandshttps://www.britannica.com/science/gastric-gland
- The gastric glands are located inside the stomach lining that play key roles in digestion. They are primarily exocrine glands and are all located under the gastric pits within the gastric mucosa. Each gastric pit contains about 3-5 gastric glands, and the cells of the gastric cells are foveolar (mucus), chief cells, and parietal cells.
- The other type of gastric gland is an endocrine gland called the pyloric gland, which secretes the hormone gastrin formed by its G-cells.
- Mucus released from the foveolar cells lines the whole stomach in order to protect the stomach lining from the acidic effects of hydrochloric acid released from other cells in the glands.
- The cardiac glands are located in the cardia of the stomach, which is adjacent to the heart. Moreover, the the cardia is the region that encloses the opening where the oesophagus connects to the stomach. They mainly release mucus, and are the least abundant compared to other gastric glands and are positioned shallowly in the mucosa. There are 2 types of cardiac glands: simple tubular with short ducts or compound racemose similar in appearance to the duodenal Bruner's glands.
- The fundic glands (or oxyntic glands) are located in the fundus and body of the stomach. They have the appearance of a virtually straight tube, at least 2 of which open into a single duct. They are described as oxyntic because they secrete hydrochloric acid (HCl) and intrinsic factor.
- The pyloric glands are located in the antrum of the pylorus, where they release gastrin produced by their G cells.
- The gastric glands contains several different types of cells, which include chief cells, enterochromaffin-like cells (ECLs), foveolar cells, G cells, parietal cells, etc.
- Foveolar cell = These cells produce mucus that covers the stomach's interior, which protects it from the corrosive nature of gastric acid. They line the gastric mucosa.
- Mucous Neck Cell = These cells are located within gastric glands, which are interspersed between parietal cells. They are shorter than surface mucous cells and contain lesser mucin granules in their apical surface.
- Chief cells (Peptic cells / zymogen cells) = These cells are located in the basal areas of the gland. They secrete proenzymes or zymogens, such as pepsinogen (precursor to pepsin), prorennin (precursor to rennin or chymosin). Prorennin is released in young mammals but not in adult mammals. Chief cells can also gastric lipase, which contributes to fat digestion.
- Parietal cells (oxyntic cells) are the most abundant cells on the side walls of the gastric gland. They release hydrochloric acid, which is the main component of gastric acid.
- In order for gastric acid to be easily available for the stomach, the parietal cells has be located on the stomach wall. They contain secretory networks of fine channels called canaliculi, which can protrude and enter all of the regions of the gastric-pit lumen.
- Parietal cells also release the castle's intrinsic factor, which is a glycoprotein essential for the absorption of vitamin B12.
- They create and secrete bicarbonate ions in response to the release of histamine from the nearby ECLs, thus playing an important role in the pH buffering system.
- Enteroendocine cells (Argentaffin cells) = They exist in the basal areas of the gastric glands, which differentiates into 3 cell types:
- Enterochromaffin-like cells (ECL cells) = These cells secrete histamine and serotonin when the stomach's pH reaches a high level (~ over 7.6). The release of gastrin from G cells stimulates the release of histamine, which augments the production and release of HCL from the parietal cells to the bloodstream and proteins to thte stomach lumen. If the stomach pH becomes more acidic (i.e. reduces in pH), the ECLs halt their release of histamine.
- G cells = These cells release a hormone called gastrin, which stimulates the gastric glands release gastric acid. These cells are typically located in pyloric glands in the antrum of the pylorus, though some can be located in the duodenum and other tissues. Since the gastric pits of these glands are deeper than other gastric pits, the gastrin is secreted into the bloodstream rather than the lumen.
- D-cells = They release somatostatin, which suppress the release of hormones from the digestive tract.
(a) Foveolar cell
Microanatomy
- Foveolar cells line the surface of the stomach's interior, the gastric pits, and the neck of the gastric glands. They compose a simple columnar epithelium in order to form a single cell layer with a height greater than its width.
- Surface mucous cells line the surface and gastric pits, while the mucous neck cells line the neck of gastric glands along parietal cells.
- Surface mucous cells contain an abundance of mucin granules in their apical surface and protrude microvilli into the lumen of the stomach. Mucins are large glycoproteins that provide the mucus its gel-like properties.
- Under the mucin granules are typical organelles of a cell such as the nucleus, Golgi apparatus and rough endoplasmic reticulum.
- The mucous neck cells situated within gastric glands are shorter than their adjacent counterpart and contain less mucin granules in their apical surface.
Functions of foveolar cells
- It produces mucus and bicarbonate icons (HCO3-) to stop the stomach from digesting itself, as well as allow the acid above pH of 4 to permeate the lining and vice versa for acid below pH of 4.
- The latter process is known as 'viscous fingering', which allows the foveolar cells to release high levels of acid that doesn't rebound once it reaches the stomach's lumen.
(b) Gastric Chief cell
- A gastric chief cell, peptic cell, or gastric zymogenic cell is a type of gastric gland cell that produces pepsinogen and gastric lipase. It plays key roles in the release of chymosin in ruminant animals and humans. They are situated deep in the mucosal layer of the stomach lining, particularly in the fundus and body of the stomach.
- When chief cells are stimulated by a number of factors, such as acetylcholine from the vagus nerve or acidic conditions in the stomach, they secrete the zymogen (enzyme precursor) pepsinogen.
- It serves in conjunction with parietal cell, which produces gastric acid and converts the pepsinogen into pepsin.
(c) Parietal cell
- Known as oxyntic cells, parietal cells are epithelial cells in the stomach that release hydrochloric acid (HCl) and intrinsic factor. These cells are found in the gastric glands, located in the lining of the fundus and body segments of the stomach.
- Gastric parietal cells contain a deep and small channel called a canaliculus to increase the cell's surface area. The number of canaliculus determines how dynamic the parietal cell membrane is and how much it can secrete.
- Sahoo, Gu & Zhang (2017) found the canalicular precursors, or tubulovesicles fuse together to increase the surface area of the parietal cell's membrane. In contrast, the reciprocal endocytosis of the canaliculi reduces the surface area of the membrane.
What are the functions of parietal cells?

i. Secretes hydrochloric acid
- The reaction of carbon dioxide and water is catalysed by carbonic anhydrase to create carbonic acid, which subsequently dissociates to yield hydrogen ions.
- The bicarbonate ion (HCO3-) is then exchanged for a chloride ion (Cl-) on the basal side of the cell and the bicarbonate diffuses into the venous blood, which results in an alkaline tide event.
- Potassium (K+) and chloride (Cl-) ions then diffuse into the canaliculi.
- Hydrogen ions are driven out of the cell into the canaliculi in exchange for K+ ions, via the H+/K+-ATPase. Activated parietal cells have increased levels of H+/K+-ATPases on the luminal side by fusion of tubulovesicles.
- The cellular export of H+ ions results in the maintenance of the acidic environment in the gastric lumen.
- The acidic environment augments the denaturing of ingested proteins as part of the digestion of food. When the proteins denature (unfold), it reveals the peptide bonds connecting the amino acids.
- Gastric hydrochloric acid simultaneously cleaves pepsinogen into pepsin (an endopeptidase) that breaks up the peptide bonds, a process called proteolysis.
ii. Regulates chemical stimuli
Parietal cells produce acid in response to the following stimuli:
- Histamine - This chemical stimulates H2 histamine receptors.
- Acetylcholine (ACh) = Produced from parasympathetic nerve activity via the vagus nerve and enteric nervous system, which stimulates M3 receptors.
- Gastrin = This chemical activates CCK2 receptors, as well as triggers histamine release by local ECL cells.
- When H2 receptors are activated by histamine, this increases the intracellular cAMP levels.
- Simultaneously, the activation of M3 receptors by acetylcholine and the CCK2 receptors by gastrin increases intracellular calcium levels.
- The elevated cAMP levels leads to increases in protein kinase A (PKA), which then phosphorylates proteins involved in the transport of H+/K+-ATPase from the cytoplasm to the cell membrane.
- This leads to the resorption of potassium ions and the release of hydrogen ions, which reduces the pH of the secreted fluid by about 0.8.
- Gastrin indirectly triggers the release of acid from parietal cells, which stimulates histamine production in ECL cells, which subsequently triggers parietal cells via the release of histamine and stimulation of H2 receptors.
- Kleveland et al. (1987) found gastrin itself has no effect on the maximum secretion of gastric acid stimulated by histamine. The effect of acetylcholine, gastrin and histamine is regarded as synergistic, meaning the effect of several simultaneous stimuli is more than the combined effects of all individual stimuli.
iii. Secretes intrinsic factor
- Parietal cells secrete a glycoprotein called intrinsic factor, which plays a key role in the absorption of vitamin B12 in the diet.
- When an autoimmune response destroys gastric parietal cells, it hinders the synthesis of intrinsic factor. This subsequently reduces the absorption of vitamin B12, which can lead a number of diseases such as megaloblastic anaemia (vitamin B12 deficiency), pernicious anaemia, and atrophic gastritis (inability to absorb vitamin B12).
(d) Goblet cell

- Goblet cells are simple columnar epithelial cells that produce mucins such as mucin 5AC. They primarily secret vesicles into a duct (merocrine method), however they might switch to the apocrine method of secretion during times of stress.
- These cells were first observed by Henle in 1837 in his study of the lining of the small intestine.
- In 1857, Leydig found mucus was secreted by these goblet cells during his study of the epidermis of fish.
- In 1867, Schulze named these cells goblet cells because they were shaped like a goblet. The apical part of the goblet cell is shaped like a cup because it is dilated by numerous granules filled with mucus. On the other hand, the basal part doesn't contain any granules and it is shaped like a stem.
- The goblet cell is polarised with the nucleus and other organelles situated at the base of the goblet cell, whereas secretory granules consisting of mucin are located at the apical surface.
- Goblet cells are mainly found in the reproductive, respiratory and gastrointestinal tracts and are surrounded by other columnar cells.
Describe the structure of the goblet cell
- Goblet cells are found throughout the epithelial lining of organs, such as the intestinal and respiratory tracts. Goblet cells are located inside the trachea, bronchi, and larger bronchioles in the respiratory tract, as well as the small intestine, the large intestine, and the conjunctiva in the upper eyelid. For instance, goblet cells in the conjunctiva provide mucin in tears and onto the ocular surface.
- Their average height is larger than their width by a factor of 4. Large mucin granules usually displace the cytoplasm of the cell body towards the basal end. They accumulate near the apical surface of the goblet cell along the Golgi apparatus, which is situated between the between the granules and the nucleus.
- This presents the basal aspect of the goblet cell a basophilic staining due to the presence of nucleic acids within the nucleus and the rough endoplasmic reticulum staining with haematoxylin.
Describe the functions of goblet cells
- Goblet cells primarily release mucus to protect the mucous membrane from the acidic environment. The mucus contains glycoproteins called mucins, which are created primarily by carbohydrates. Mucin has gel-like properties due to tis glycans attracting numerous water particles.
- There is a 200 µm thick layer of mucus on the inner surface of the human intestine that lubricates and protects of the intestinal wall. MUC2 is commonly found in the intestine, while MUC5AC and MUC5B are prevalent in the human airway.
- In the airway, the cilia of the respiratory epithelium brushes off the mucus, in a process known as mucociliary clearance. This process drives mucus out of the lungs and into the pharynx, which ultimately removes debris and pathogens from the airway.
- Adler et al. (2013) found mucins are continuously produced and released by goblet cells to repair and replace the existing mucus layer. Johansson et al. (2013) found mucins are stored in granules prior to their release from the goblet cells to the lumen of the organ.
- Rubin (2013) reported that mucus secretion is typically stimulated by the presence of irritants such as dust and smoke, as well as viruses and bacteria.
- Overexpression of MUC5AC as well as the excessive and rapid secretion of extremely thick mucus that can't be easily removed by cilia or coughing results in goblet cell anomalies seen in asthma patients. In addition to airway constriction, the accumulation of thick mucus results in clogged airways, which poses serious health problems if not treated early.
- Oral tolerance: This process is defined as the immune system being inhibited from responding to antigens or peptides arising in food products in the bloodstream. McDole et al. (2012) discovered CD103-expressing dendritic cells of the lamina propria is involved in the initiation of oral tolerance (probably by stimulating the differentiation of regulatory T cells. The study suggests the goblet cells serve to preferentially deliver antigen to these CD103+ dendritic cells.
iv. Gene and protein expression
- Uhlén et al. (2015) estimated about 20,000 protein coding genes are expressed in human cells and approximately 70% of these genes are expressed in the normal stomach. Roughly 150 of these genes are more specifically expressed in the stomach compared to other organs, with only about 20 genes with high specificity.
- The corresponding specific proteins expressed in the stomach are primarily involved in producing a suitable environment for handling the process of food digestion for the uptake of nutrients.
- Gremel et al. (2015) listed proteins highly specific to the stomach include GKN1 (expressed in mucosa), pepsinogen PGC and the lipase LIPF (expressed in chief cells), and gastric ATPase ATP4A and gastric intrinsic factor GIF (expressed in parietal cells).
Describe the development of the stomach
- In early human embryogenesis, the ventral region of the embryo borders the yolk sac. During the 3rd week of development, the growing embryo starts to surround regions of the sac.
- The enveloped parts of the sac start to form the basis for the adult gastrointestinal tract. The sac subsequently becomes surrounded by a network of vitelline arteries and veins.
- Over time, these vitelline arteries consolidate into the 3 main arteries that provide blood to the developing gastrointestinal tract: the coeliac artery, the superior mesenteric artery, and the inferior mesenteric artery. These 3 arteries supply to different parts of the gut: the foregut, the midgut, and the hindgut. The surrounded sac ultimately forms the primitive gut.
- Schoenwolf (2009) discovered that certain segments of this gut start to differentiate into the organs of the gastrointestinal tract, e.g. stomach forms from the foregut.
Describe the functions of the stomach
i. Digestion
- Mechanical
- When food enters the stomach via the oesophagus, it triggers a mixing wave at internals of about 20 seconds. This mixing wave is a type of peristalsis that combines and softens the food with gastric juices to produce chyme.
- The first mixing waves are relatively mild, which is subsequently followed by stronger waves. This process begins at the body of the stomach and increases in force as the chyme reaches the pylorus.
- As chyme enters the pylorus, it becomes filtered with only liquids and small food particles passing through a slightly ajar pyloric sphincter. Known as gastric emptying, rhythmic mixing waves propel about 3 mL of chyme every cycle through the pyloric sphincter and into the duodenum.
- If excess chyme is released at one time, it would overload the small intestine's capacity to handle it. Therefore, some of the chyme is forced back into the stomach's body, where it resumes mixing. This process occurs again when the next mixing waves push more chyme into the duodenum.
- When chyme enters the duodenum, it activates receptors that inhibit gastric secretion. This blocks any excess chyme from being released by the stomach before it can be processed by the duodenum.
- Chemical
- Both undigested food and gases released during chemical digestion are stored in the fundus of the stomach. When food reaches the fundus of the stomach, it gets disintegrated by salivary amylase until the food starts combining with the acidic chyme.
- Then mixing waves integrates the food with the acidic chyme, which inactivates salivary amylase and activates lingual lipase Subsequently, lingual lipase starts disintegrating triglycerides into free fatty acids, as well as mono-and diglycerides. Hydrochloric acid and pepsin in the stomach starts to disintegrate proteins.
- Betwen 2 to 4 hours after a meal is consumed, the contents of the stomach are entirely deposited into the duodenum.
- High carbohydrate foods are discharged the fastest, followed by high-protein foods, whereby high-triglyceride food stay in the stomach for the longest time.
- Because enzymes in the small intestine digest fats gradually, food can remain in the stomach for 6 hours or longer when the duodenum is processing fatty chyme.
ii. Absorption
Some small molecules are absorbed into the body via the stomach through its lining. They include:
-- Water (if dehydrated)
-- Medication (e.g. aspirin)
-- Amino acids
-- 10-20% of ethanol (e.g. from alcoholic drinks)
-- Caffeine
-- Some water-soluble vitamins (e.g. vitamin B12 thanks to intrinsic factor produced by the parietal cells of the stomach.
iii. Control of secretion and motility
Chyme gradually flows from the stomach into the duodenum via the pyloric sphincter through coordinated peristalsis. Both the autonomic nervous system and a number of digestive hormones of the digestive system regulate the movement and the flow of chemicals into the stomach.
- Gastrin = This hormone increases the release of hydrochloric acid (HCl) from the parietal cells and pepsinogen from chief cells in the stomach, as well as increase motility in the stomach. Furthermore, G cells release gastrin in response to distension of the antrum and the presence of digestive products (especially undigested proteins). Gastrin is inhibited when the pH of the stomach reduces to 4 or less, as well as the presence of somatostatin.
- Cholecystokinin (CCK) = This hormone primarily causes contractions of the gall bladder, as well as reduces gastric emptying and elevates secretion of pancreatic juice. Since the pancreatic juice is an alkaline chemical, it neutralises the chyme. I-cells in the mucosal epithelium of the small intestine synthesises CCK.
- Secretin = This hormone mainly acts on the pancreas, as well as decreases acid release in the stomach. I is synthesised by S-cells, located in the duodenal and jejunal mucosa.
- Gastric inhibitory peptide (GIP) = This chemical reduces both gastric acid release and motility. GIP is synthesised by K-cells, located in the duodenal and jejunal mucosa.
- Enteroglucagon = This hormone reduces both gastric acid and motility of the stomach.
Besides gastrin, the above hormones all serve to deactivate the function of the stomach. This occurs in response to food products in the liver and gall bladder, which haven't yet been absorbed. The stomach forces the food into the small intestine only when the intestine is empty. If the intestine is full and in the process of digesting food, the stomach serves to store the food.
iv. Other
- EGF
- Epidermal growth factor (EGF) typically promotes cellular proliferation, differentiation, and survival. It is a polypeptide that is found in numerous human tissues including the submandibular gland, and the parotid gland.
- Salivary EGF plays an physiological essential role in maintaining oro-esophageal and gastric tissue integrity. The effects of salivary EGF include inhibition of gastric acid secretion, healing of oral and gastroesophageal ulcers, stimulation of DNA synthesis, and mucosal protection from chemicals that cause intraluminal injuries. Those chemicals include bile acids, gastric acid, pepsin, and trypsin, as well as from physical, chemical, and bacterial agents.
- Nutrition Sensor
- The human stomach contains receptors that respond to sodium glutamate, which transfers information to the lateral hypothalamus and limbic system in the brain. Uematsu et al. (2010) described this process as a palatability signal through the vagus nerve.
- Studies found the stomach can perceive a number of molecules such as carbohydrates, glucose, fats and proteins. It senses them independently of tongue and oral taste receptors. Araujo et al. (2008) found this allows the brain to associate nutritional value of foods with their tastes.
- Thyrogastric syndrome
- First described in the 1960s, thyrogastric syndrome defined the link between thyroid disease and chronic gastritis. Doniach et al. (1965) coined the term to describe the thyroid autoantibodies or autoimmune thyroid disease in patients with pernicious anaemia, which is a late clinical stage of atrophic gastritis.
- In 1993, Venturi et al. (1993) conducted a thorough investigation of the stomach and thyroid, and discovered that the thyroid has embryonic and phylogenetic roots to a primitive stomach. Thereby the thyroid cells, such as primitive gastroenteric cells, migrated and specialised in the uptake of iodide, as well as in storage and elaboration of iodine compounds during the evolution of vertebrates.
- Researchers found the stomach and thyroid share similarities in abilities focused on iodine, as well as other abilities such as digestion and readsorption, cell polarity and apical microvilli, organ-specific antigens and associated autoimmune diseases, release of glycoproteins (e.g. thyroglobulin and mucin) and peptide hormones, and production of iodotyrosines by peroxidase.
12. Spleen
The spleen is an organ in virtually all vertebrates that serves as a large blood filter. The word spleen originates from the Ancient Greek σπλήν (splḗn).
Anatomy of the spleen
- The spleen is located under the diaphragm's left side, as well as the 9th, 10th and 11th ribs. It contains a smooth, convex surface that faces the diaphragm.
- The other side of the spleen is segmented by a ridge into 2 parts: an anterior gastric section, and a posterior renal section.
- The gastric surface is broad and concave, facing forwards, upwards, and towards the centre. Moreover, this surface's front side touches the posterior wall of the stomach, and its underside touches the tail of the pancreas.
- On the other hand, the renal surface faces medially and downwards and is flatter and narrower than the gastric surface. It is located relative to the upper section of the anterior surface of the left kidney and part of the left adrenal gland.
- 4 ligaments are connected to the spleen: colicosplenic ligament, gastrosplenic ligament, phrenocolic ligament and splenorenal ligament.
Sizes of the spleen
- In healthy adult humans, the spleen is between 7 and 14 cm (between 3 and 5.5 in) in length. It is roughly 3 x 8 x 13 cm (1 x 3 x 5 in) and weighs between 28 and 230 g (between 1 and 8 oz).
- It situates between the 9th and 11th ribs on the left hand side and along the axis of the 10th rib.
90% confidence interval of spleen length by abdominal ultrasonography by height of the person (Chow et al. (2016))
i. Development
The spleen originates from mesenchymal tissue, specifically within, and from, the dorsal mesentery. Nevertheless, it shares the same same supply as the foregut organs, that is, the coeliac trunk.
ii. Nerve supply
The spleen is innervated by the splenic plexus, which links to a branch of the coeliac ganglia to the vagus nerve.
Lori et al. (2017) found the primary central nervous processes responsible for the function of the spleen is lodged in the hypothalamic-pituitary-adrenal-axis, and the brainstem, especially the subfornical organ.
iii. Blood supply
There is a long fissure located near the middle spleen called the hilum. It is the point where the gastrosplenic liament connects to, as well as the point where the splenic artery and splenic vein inserts. Furthermore, collateral blood supply is provided by the adjoining short gastric arteries. Since the spleen is part of the lymphatic system, it contains only efferent lymphatic vessels.
What are the functions of the spleen?
The spleen is composed of red pulp and white, which is divided by a marginal zone. About 76-79% of the pulps in a typical spleen are red pulps.
i. Red pulp
- The red pulp of the spleen comprises of connection tissue called the cords of Billroth and numerous splenic sinusoids filled with blood, hence giving it its red appearance. Its main function is to filter the blood of antigens, microorganisms, and defective red blood cells. Furthermore, it consists of different types of cells, such as granulocytes, platelets, plasma and red blood cells.
- The red pulp serves as a large reservoir for monocytes, which are located in clusters in the Billroth's cords. The population of monocytes in this reservoir is higher than the total number of monocytes present in circulation. These monocytes can be instantly recruited to depart the spleen and aid in eliminating infections.
- The splenic sinusoids are wide vessels that drain into pulp veins, which subsequently drain into trabecular veins. As blood cells enter the spleen, they are mechanically filtered at orifices in the endothelium lining the sinusoids.
- If abnormal or aged red cells try to wriggle through the narrow intercellular spaces, they become severely damaged. Thus, macrophages subsequently consume these damaged red cells in the red pulp.
- Furthermore, the sinusoids also filter out cellular debris, or particles that could clutter up the bloodstream.
What cells are found in red pulp?
Red pulp comprises of a tightly packed network of fine reticular fibre that is continuous with those of the splenic trabeculae.
Inside the meshes of the reticulum:
- White blood cells are situated in red pulp at higher levels than in circulation.
- Splenic cells are large rounded cells that move like amoeba, and tend to contain pigment and red-blood corpuscles inside them.
- Each of the cells of the reticulum contain a round or oval nucleus, as well as pigment granules in their cytoplasm. However, they don't stain deeply with carmine, and to that extent differ from the cells of the Malpighian corpuscles.
- Nucleated red blood cells and macrophages containing a compound nucleus or many nuclei in a young spleen.
Describe red pulp macrophages
- Red pulp macrophages (RPMs) are mononuclear phagocytes situated inside the spleen that play key roles in maintenance of blood homeostasis. They achieve this by undergoing phagocytosis of injured and senescent erythrocytes and blood-borne particulates.
- A number of cell-intrinsic and cell-extrinsic factors have been identified to modulate the development and survival of RPMs, which include heme oxygenase-1, IRF8/4, M-CSF and Spi-C.
- Kurotaki et al. (2015) found that RPMs can trigger regulatory T cell differentiation by expressing transforming growth factor-β. Moreover, they can release type-1 interferons in response to parasitic infections.
- Blood flows from the arteries to a bunch of red pulp cords called Billroth's cords, which are composed of fibroblasts and reticular fibres that serve as an open blood system without an endothelial lining.
- Situated within these cords are the F4/80+ macrophages, which are associated with the reticular cells of these cords and are collectively referred to as red pulp macrophages (RPMs).
- Blood then flows from the Billroth's cords to the venous sinuses of the red pulp, which are lined with discontinuous endothelium and stress fibres that stretch under the basal plasma membrane, parallel to the cellular axis. The combined positioning of the stress fibres and the parallel sinus endothelial cells propel blood in the red pulp through slits created by the stress fibres.
- However, ageing old red blood cells may get stuck in the red pulp cords because their membranes are less flexible, and thus get subsequently phagocytosed by the red pulp macrophages. This process is called erythrophagocytosis, which plays a key role in the turnover of red blood cells and the recycling of iron particles by the red pulp macrophages.
- The iron is either released by the red pulp macrophages or stored in the erythrocyte itself in the form of ferritin or hemosiderin (an insoluble complex of partially degraded ferritin).
- Moreover, the RPMs scavenge a complex of haemoglobin (released from erythrocytes disintegrated intravascularly throughout the body) and haptoglobin to collect iron, through endocytosis by CD163. The iron stored in RPMs is released according to the needs of the bone marrow.
ii. White pulp
About 25% of splenic tissue is white pulp, because it appears whiter than the surrounding red pulp on gross section. Functions of white pulp include:
- The periarteriolar lymphoid sheaths (PALs) contain T-lymphocytes, and are usually associated with the arteriole supply of the spleen.
- Lymph follicles containing B lymphocytes undergoing mitosis are found between the PALS and the marginal zone on the border of red pulp.
- IgM and IgG2 are formed in the marginal zone, which serve to opsonise extracellular organisms, as well as encapsulate bacteria.
- Located between the white pulp and the red pulp is the marginal zone, which is quite a distance from the central arteriole. It contains antigen-presenting cells (APCs), such as dendritic cells and white pulp macrophages e.g. metallophilic macrophages.
- The T-cell zone and B-cell follicles contain separate macrophage populations, but it is not known where these macrophages originated and how long their lifespan is.
- In the B-cell follicles, the macrophages serve to remove the apoptotic B cells resulting from the germinal centre reaction during somatic hypermutation and isotype switching processes.
- In the germinal centre, macrophages digest any B-cells that died of apoptosis due to failure to form their appropriate receptors. Phosphatidylserine is expressed on the surface of apoptotic B-cells in order to recognised by the receptors on the surface of macrophages, hence trigger the engulfment and disintegration by macrophages.
- Macrophages express receptors such as tyrosine kinase Mer, the milk fat globule epidermal growth factor 8 and Tim-4, all of which augment the engulfment of apoptotic cells into the macrophages.
- The roles of macrophages in the T-cell zone of the white pulp aren't well understood. den Haan, Joke & Kraal (2012) suggested these macrophages are involved in antigen presentation or the elimination of dying lymphocytes due to their proximity to T-cells.
iii. Other functions
- Production of all types of blood cells during foetal development.
- Produces properdin, opsonins, and tuftsin.
- Secretes neutrophils as a result of myocardial infarction.
- Formation of red blood cells = Although the bone marrow is main location of haemotopoiesis in an adult human, the spleen elicits essential haematopoietic functions up until the 5th month of gestation. Erythropoietic functions usually terminate after birth, except in some haemotologic disorders. Since the spleen is a major lymphoid organ in the reticuloendothelial system, it maintains the ability to form lymphocytes.
- Storage of red blood cells, lymphocytes and other biological elements: The spleen of horses stores approximately 30% of the red blood cells and releases them when required. A human's spleen stores up to 240 mL of red blood cells and releases them in cases of hypoxia and hypovolemia. In addition, the spleen can store platelets and remove any aged platelets from circulation, as well as store about 25% of lymphocytes.
13. Liver
Describe the structure of the liver
- The liver is a major metabolic organ that is found only in vertebrate animals. It is dark reddish brown in colour and it shaped like a large wedge comprised of two lobes of unequal size and shape. It is regarded as both the largest gland and the heaviest internal organ in the human body.
- The prefix hepat- is derived from Greek word ἡπατο-, meaning the liver.
- An average adult human liver typically weighs about 1.5 kg (3.3 lb) and is about 15 cm (6 in) wide. An adult man's liver weighs between 0.97 and 1.86 kg (between 2.14 and 4.10 lb), whereas an adult woman's liver weighs between 0.6 kg and 1.77 kg (between 1.32 and 3.90 lb).
- Tortora & Derrickson (2008) described the position of the liver as in the right upper quadrant of the abdominal cavity, sitting underneath the diaphragm, to the stomach's right, and superimposing the gallbladder.
- The hepatic artery supply oxygenated blood from the aorta via the celiac trunk to the liver. Then blood rich in digested nutrients from the GIT, spleen and pancreas is drained from the liver by the portal vein. These blood vessels split into small capillaries called liver sinusoids, which subsequently lead to hepatic lobules.
- Hepatic lobules comprise of millions of hepatic cells (hepatocytes), which are the basic metabolic cells. The lobules are connected by a dense, fine, fibroelastic, irregular connective tissue layer stretching from the fibrous capsule over the whole liver called Glisson's capsule (named after British doctor Francis Glisson). Glisson's capsule reaches into the liver by being concomitant with blood vessels, ducts, and nerves at the hepatic hilum.
- Beside the bare area, the entire surface of the liver is coated in a layer of serous membrane derived from the peritoneum, which sticks to the inner Glisson's capsule.
a. Gross anatomy
-- Lobes
- The liver is divided into 2 sections when viewed from above: a left lobe and a right lobe. In addition, it is divided into 4 sections when viewed from below - left lobe, right lobe, caudate lobe, and quadrate lobe.
- The falciform ligament superficially divides the liver into the left and right lobes. From underneath, the caudate and quadrate lobes are situated between the left and right lobes, one in front of the other.
- Renz & Kinkhabwala (2014) described an imaginary line from the left of the vena cava and entirely forward to split the liver and gallbladder into 2 halves, which is known as Cantlie's line. The ligamentum venosum and the round ligament further segments the left lobe into 2 sections.
- The porta hepatis divides this left section into 4 segments, which are numbered beginning at the caudate lobe as I (1) in a counter-clockwise direction. A parietal view of the liver displays 7 segments, because the 8th segment is only visible when viewing the liver viscerally.
- The left lobe is located in the epigastric, and the left hypochondriac regions of the abdomen. Its upper surface is slightly convex and is positioned onto the diaphragm, whereas its under surface has the gastric impression and omental tuberosity.
ii. Right lobe
- The right lobe is about 6 times larger than the left lobe. It settles in the right hypochondrium, on its posterior surface by the ligamentum venosum for the cranial half and by the ligamentum teres hepatis for the caudal half. The ligamentum teres hepatis revolves around the inferior margin of the liver to end up ventral in the falciform ligament.
- The middle hepatic vein functionally divides the right lobe from the left lobe. The falciform ligament functionally divides the lateral and medial parts of the left hepatic lobe.
- The right is quadrilateral in shape to a certain extent. Its posterior and under surfaces are demarcated by 3 fossae: the portal vein fossa, the gallbladder fossa and the inferior vena cava fossa. They separate the right lobe into 2 smaller lobe on its left posterior region: the quadrate lobe and the caudate lobe.
iii. Quadrate lobe
- The quadrate lobe is located on the undersurface of the medial section of the left lobe (Couinaud segment IVb). It is confined behind by the porta hepatis, in front by the anterior margin of the liver, on the left by the fossa for the umbilical vein, and on the right by the fossa for the gallbladder.
- It takes up an oblong appearance, with a larger antero-posterior diameter than its transverse diameter.
iv. Caudate lobe
- The caudate lobe (posterior hepatic segment I) is located upon the posterosuperior surface of the liver on the right lobe. It is confined below by the porta hepatis, on the left by the ductus venosus fossa and the ligamentum venosum, and on the right by the fossa for the inferior vena cava.
- The caudate lobe is positioned vertically and faces rearwards, is slightly concave in the transverse direction and its horizontal length being shorter than its vertical length. It is located behind the porta, separating the gallbladder fossa from the inferior vena cava fossa.
- The caudate lobe is named after the tail-shaped hepatic tissue papillary process of the liver, which originates from its left side. It is derived from the Latin word 'cauda' meaning "tail".
- It also contains a caudate process (that isn't shaped like a tail) protruding from its right side, providing surface continuity between the caudate lobe and the visceral surface of the anatomical right lobe. This caudate process is a little rise of the hepatic substance that stretches obliquely and laterally, from the lower extremity of the caudate lobe to the undersurface of the right lobe.
- Blood supply to the caudate lobe is mainly provided by the caudate arteries, which arise from the left, middle and right hepatic arteries that are linked to each other. In addition, the caudate lobe receives blood supply from the left and right branches of the portal vein too. Blood from the caudate lobe is drained through the short hepatic veins, which drain directly into the inferior vena cava (IVC) because of its proximity to the IVC.
-- Surfaces
- On the diaphragmatic surface of the liver, there is a thin, double-layered membrane called the peritoneum to minimise friction against other organs. In addition, there is a triangular bare area where the liver connects to the diaphragm. This surface envelops the convex shape of the 2 lobes where it accommodates the shape of the diaphragm.
- The peritoneum turns over on itself to create the falciform ligament, the left and triangular ligaments. McMinn (2003) found these peritoneal ligaments have no relation to the anatomic ligaments in the joints, and the left and right triangular ligaments have no known functional significance. The falciform ligament connects the liver to ther posterior aspect of the anterior body wall.
- The visceral (inferior) surface is concave and bumpy because it is entirely covered in peritoneum except the point where it connects to the gallbladder and porta hepatis. The fossa of the gallbladder is located to the right of the quadrate lobe, which is filled up by the gallbladder with its cystic duct adjacent to the right end of porta hepatis.
-- Impressions
- There are a number of impressions of the liver's surface that contain several adjacent structures and organs. There are 2 impressions located below the right lobe and on the right of the gallbladder fossa, with one situated behind the other, with a ridge separating them.
- Skandalakis et al. (2009) described the impression in front as shallow and colic, formed by the hepatic flexure. In contrast, the impression in the back is described as deeper and renal, which accommodates a section of the right kidney and a section of the suprarenal gland.
- The suprarenal impression is a small, triangular, concave region on the liver's surface, which is situated near to the right of the fossa. In addition, it is located between the bare area and the caudate lobe, and immediately above the renal impression. The broader aspect of the suprarenal impression lacks peritoneum and it embeds the right suprarenal gland.
- There is a 3rd impression located medial to the renal impression, near the neck of the gall bladder. This impression is created by the descending aspect of the duodenum, known as the duodenal impression.
- The inferior surface of the left lobe appears behind and to the left of the gastric impression. This impression forms over the upper front surface of the stomach, with the tuber omentale located to the right. The tuber omentale slots into the concavity of the lesser curvature of the stomach and situates in front of the anterior layer of the lesser omentum.
b. Microscopie anatomy
- Microscopically, each live lobe consists of hepatic lobules, which are somewhat hexagonal, and comprise of plates full of hepatocytes, and sinusoids diffusing from a central vein towards an imaginary perimeter of interlobular portal triads. The central vein links up with the hepatic vein to transport blood away from the liver.
- A distinct part of a lobule is the portal triad, which runs along each of the lobule's corners. The portal triad contains the hepatic artery, the portal vein, and the common bile duct.
- Under histological analysis, there are 2 major types of live cell: parenchymal cells and non-parenchymal cells. About 70-85% of the liver's volume is filled with parenchymal hepatocytes, whereas about 6.5% of the liver's volume is filled with non-parenchymal cells despite accounting for about 40% of the liver cells.
- Gillian Pocock (2006) found the liver sinusoids are lined with sinusoidal endothelial cells and phagocytic Kupffer cells. Kmieć (2001) discovered hepatic stellate cells are non-parenchymal cells in the perisinusoidal space, situated between a sinusoid and hepatocyte. Furthermore, intrahepatic lymphocytes are also present in the sinusoidal lumen.
c. Functional anatomy
- The central area or hepatic hilum consists of the main opening called the porta hepatis, which comprises of the common bile duct, the common hepatic artery, and the opening of the portal vein.
- The artery, duct and vein split into left and right branches, and the segments of the liver supplied by these branches comprise the functional left and right lobes.
- The functional left and right lobes are divided by an imaginary plane called Cantlie's line, linking the gallbladder fossa to the inferior vena cava. In addition, the middle hepatic vein demarcates the true left and right lobes.
- The right lobe is further split into anterior and posterior sections by the right hepatic vein. Furthermore, the left lobe is split into the medial and lateral sections by the left hepatic vein.
- The hilum of the liver consist of 3 plates that comprise of the bile ducts and blood vessels. Kawarada et al. (2000) labelled these 3 plates as the cystic plate, hilar plate, and the umbilical plate. The entire plate system is encompassed by a sheath and is the main location of numerous anatomical variations in the liver.
d. Couinaud classification system
- The Couinaud system illustrates the functional lobes further separating into 8 subsegments situated on a transverse plane through the bifurcation of the main portal vein. Strunk et al. (2003) found the caudate lobe receives blood supply from both the left- and right-sided vascular branches.
- The Couinaud system classifies the liver into 8 functionally independent liver segments. Each segment receives its own blood supply, as well as blood and biliary drainage. The centre of each segment contains the branches of the bile duct, hepatic artery, and portal vein.
- In the periphery of each segment, there is blood drainage through the hepatic veins. The classification system depicts the functional units (labelled I to VIII) of the liver according to their vascular supply.
- For instance, the caudate lobe is unit I (1) since it receives blodd from both the left and right branches of the portal vein. In addition, the caudate lobe contains at least 1 hepatic vein that drain directly into the inferior vena cava. A 2006 study explained the other units (II to VIII) are numbered in a clockwise manner.
e. Gene and protein expression
- Uhlén et al. (2015) estimated about 60% of the 20,000 protein coding genes are expressed in a typical, adult human liver. Furthemore, there are more than 400 genes specifically expressed in the liver, with roughly 150 genes highly specific for liver tissue.
- A significant portion of the corresponding liver-specific proteins specific are primarily expressed in hepatocytes and secreted into the blood. Those liver-specific proteins comprise of plasma proteins and hepatokines, as well as liver enzymes such as HAO1 and RDH16, bile synthesis proteins such as BAAT and SLC27A5, and transporter proteins involved in drug metabolism, such as ABCB11 and SLC2A2.
- Kampf et al. (2014) listed examples of highly liver-specific proteins such as apolipoprotein A II, coagulation factors F2 and F9, complement factor related proteins, and the fibrinogen beta chain protein.
Describe the development of the liver
 |
| Timeline of mouse liver development |
This diagram illustrates mouse embryos at different stages of development with the endoderm tissue (yellow), the liver (red) and the gall bladder (green).
- The endoderm germ layer forms during gastrulation (e6.5-e7.5).
- Throughout gastrulation and early somite stages of development (e7-e8.5) the endoderm patterns along the A-P axis into foregut (fg) midgut (mg) and hindgut (hg) progenitor domains.
- Morphogenesis results in the formation of foregut and hindgut pockets as the endodermal cup transforms into a gut tube.
- By e8.5, hepatic fate is specified in a section of the ventral foregut endoderm adjacent to the heart.
- As the embryo expands, the endoderm creates a gut tube and the liver domain shifts to the midgut.
- The liver diverticulum (Id) appears by e9 and develops into an obvious liver bud (lb) by e10.
- The liver expands, and by315 hepatoblasts differentiate into hepatocytes and biliary cells.
- The final stage of liver maturation is gradual, which continues into the postnatal period.
- The development of the liver begins between the 3rd and 8th week of embryogenesis, which situates in both the ventral part of the foregut endoderm and the components of the septum transversum mesenchyme. In the human embryo, an embryonic tube called the hepatic diverticulum stretches out form the foregut into the surrounding mesenchyme.
- The mesenchyme of septum transversum stimulates this endoderm to proliferate, to bifurcate, and to develop the glandular epithelium of the liver. A section of the hepatic diverticulum carries on serving as the drainage duct of the liver, and a branch from this drainage duct forms the gallbladder.
- Hepatic competence is achieved when there are signals from the septum transversum mesenchyme, fibroblast growth factor from the developing heart and retinoic acid produced by the lateral plate mesoderm.
- The hepatic endodermal cells undergo a morphological change from columnar to pseudostratified, which culminates in the thickening of the early thick bud. Studies found this process produces a population of bipotential hepatoblasts, and hepatic stellate cells (derived from mesenchyme).
- After hepatoblasts migrate into the septum transversum mesenchyme, the hepatic architecture starts to take shape, with the first appearance of liver sinusoids and bile canaliculi.
- The liver bud then splits into lobes, with the left umbilical vein forming into the ductus venosus and the right vitelline vein forming the portal vein. Hematopoietic cells subsequently colonise the expanding liver bud, and the bipotential hepatoblasts start to differentiate into hepatocytes and epithelial cells.
- The biliary epithelial cells then differentiate from hepatoblasts surrounding the portal veins, initially forming a monolayer, and then a bilayer of cuboidal cells. In the ductal plate, a number of focal dilations appear at certain sites in the bilayer, which become enveloped by portal mesenchyme, then undergo tubulogenesis into intrahepatic bile ducts.
- Hepatoblasts that aren't abutting the portal veins instead differentiate into hepatocytes and arrange into cords lined by sinusoidal epithelial cells and bile canaliculi. When hepatoblasts have been specified into hepatocytes and undergo additional expansion, they start to gain the functions of a mature hepatocyte. Eventually, mature hepatocytes become highly polarised epithelial cells with abundant stores of glycogen.
- In the adult liver, hepatocytes aren't equivalent because each of the cell's position along the portocentrovenular axis within a liver lobule determines the expression of metabolic genes involved in drug metabolism, ammonia detoxification, carbohydrate metabolism, and bile production and secretion. Lade & Monga (2011) discovered Wnt/β-catenin plays an important role in this process.
- At birth, the liver constitutes approximately 4% of body weight and weighs an average of 120 g (4 oz). As the living human continues to develop, the liver will become heavier to 1.4–1.6 kg (3.1–3.5 lb), but decrease in proportion of body weight to 2.5-3.5%.
Foetal blood supply to the liver
- The umbilical vein primarily supplies blood to the liver and nutrients to the developing foetus. The umbilical vein enters the abdomen at the umbilicus and goes upward along the free margin of the falciform ligament to the liver's inferior surface. At this point, it connects to the left branch of the portal vein.
- The ductus venosus transports blood from the left portal vein to the left hepatic vein and subsequently to the inferior vena cava, which allows placental blood to bypass the liver. In the foetus, the infant liver receives nutrients directly from the mother via the placenta, meaning the liver doesn't serve its typical digestive processes and filtration process.
- The foetal liver secretes blood stem cells that migrate to the foetal thymus, which result in T cells. After birth, the production of blood stem cells transitions to the red bone marrow.
- After 2 to 5 days, the umbilical vein and ductus venosus are disintegated, with the former becoming the round ligament and the latter becoming the ligamentum venosum.
What are the functions of the liver?
a. Blood supply
- The hepatic portal vein supplies about 75% of the liver's blood supply, whereas the hepatic arteries supplies the remaining 25%. Venous blood is drained by the hepatic portal vein from the gastrointestinal tract, the spleen, and its related organs.
- The oxygen supply is provided equally by the hepatic portal vein and the hepatic arteries (50% each). In addition, the hepatic artery contains both alpha- and beta-adrenergic receptors that regulate blood flow through the artery partly by the splanchnic nerves.
- Blood flows through the liver sinusoids and drains into the central vein of each lobule. The central veins combine to become hepatic veins, which exit the liver and drain into the inferior vena cava.
b. Biliary flow
- Bile flows through the biliary tract and out of the liver to the first section of the small intestine called the duodenum. This bile is then accumulated in the canaliculi, which are small grooves between the walls of adjacent hepatocytes. The canaliculi extend to the edge of the lobule, where they coalesce to form bile ducts.
- The bile ducts within the liver labelled intrahepatic, whereas bile ducts outside of the liver are labelled extrahepatic. The intrahepatic ducts drain into the left and right hepatic ducts, which depart the liver at the transverse fissure, and then combine to form the common hepatic duct. The cystic duct from the gallbladder merges with the common hepatic duct to become the common bile duct.
- Bile either drains directly into the duodenum via the common bile duct, or temporarily collects in the gallbladder via the cystic duct. The common bile duct and the pancreatic duct then join up at the second section of the duodenum together at the hepatopancreatic ampulla, sometimes referred to as the ampulla of Vater.
c. Metabolism
- Carbohydrates = The liver synthesises and stores about 100g of glycogen via glycogenesis. On demand, the liver undergoes glycogenolysis to break down glycogen into glucose, which then gets released into the bloodstream. The liver also produces glucose from other molecules, such as amino acids, lactate and glycerol, by a process called gluconeogenesis. The liver undergoes glyconeogenesis to produce glycogen from lactic acid where then becomes stored until required by the body, restarting the cycle.
- Proteins: The liver synthesises all plasma proteins except Gamma-globulins and a majority of amino acids. The liver is involved in the production of clotting factors and red blood cells. Examples of clotting factors include coagulation factors I (fibrinogen), II (prothrombin), V, VII, VIII, IX, X, XI, XII, XIII, protein C, protein S and antithrombin. Furthermore, the liver produces a glycoprotein hormone called thrombopoietin, which regulates platelet production by the bone marrow.
- Lipids = The liver is the main organ in the synthesis of cholersterol, lipogenesis, the production of triglycerides and lipoproteins. The liver produces and secretes bile to emulsify fats and aid in the absorption of vitamin K. Bile either drains into the duodenum or stores in the gallbladder. The liver also creates a polypeptide protein hormone called insulin-like growth factor 1, which is involved in childhood growth and elicits anabolic effects in adults.
d. Breaking down molecules
- The liver is involved in the disintegration of insulin and other hormones, bilirubin via glucuronidation, waste products, toxic substances and a majority of pharmaceutical products or drugs.
- Drug metabolism may lead to toxication due to the metabolite being more toxic than its precursor. It is preferable that the toxins are conjugated to be excreted in bile or urine.
- The liver is the site where ammonia is converted into urea as part of the ornithine or urea cycle, and the urea is subsequently excreted in the urine.
e. Blood reservoir
- Due to the liver's ability to expand, a large amount of blood is stored in its blood vessels. Its typical blood volume (including the hepatic veins and the hepatic sinuses) is about 450 millilitres (~10% of the body's total blood volume).
- As pressure increases in the right atrium, it results in backpressure in the liver. This leads to expansion of the liver, and between 0.5 and 1 litre of additional blood is occasionally stored in the hepatic veins and sinuses. This phenomenon may occur in cardiac failure due to peripheral congestion.
- Therefore, the liver is a large venous organ capable of expanding and serving as an essential blood reservoir during times of excess blood volume, as well as providing additional blood during times of reduced blood volume.
f. Lymph production
- Since there are permeable pores in the hepatic sinusoids, both fluids and proteins can traverse into the perisinusoidal space. The lymph being drained from the liver typically has a protein concentration of around 6 g/dl, which is slightly less than the protein concentration of plasma.
- Furthermore, the formation of lymph occurs at a greater rate due to the high permeability of the sinusoid epithelium. Thus, around 50% of all the lymph produced in the body under rest occurs in the liver.
g. Other functions
- Storage of various substances, such as vitamin A, vitamin B12, vitamin D, vitamin E, vitamin K, copper, cobalt, iron, molybdenum, zinc, etc.
- Haemopoiesis = Formation of red blood cells and white blood cells during the embryonic stage and the first trimester until the 32nd week of gestation.
- The Kupffer cells of the liver are phagocytic cells, which aid in phagocytosis of dead blood cells and bacteria from the blood, purifying the blood.
- Immunologically active cells in the liver's mononuclear phagocytes system scavenges for antigens carried to the liver via the portal system.
- Production of a protein called albumin, which helps maintain the oncotic pressure, and serves as a carrier of fatty acids and steroid hormones.
- Synthesis of a hormone called angiotensinogen, which elevates blood pressure when activated by renin. Renin is an enzyme released by the kidney when it senses low blood pressure.
- Production of the enzyme catalase to break down hydrogen peroxide into water and oxygen.
i. Bile
- Also known as gall, bile (from Latin bilis) is a yellow-green fluid produced by the liver of most vertebrates that support digestion of lipids in the small intestine.
- In the human liver, bile is comprised of 97-98% water, 0.7% bile salts, 0.51% fats (cholesterol, fatty acids, and lecithin), 0.2% bilirubin, and 200 meq/L inorganic salts.
- The 2 pigments of bile are bilirubin and its oxidised form biliverdin, which are yellow and green respectively. When those pigments are combined, they become the brown colour of faeces. Between 400 and 800 mm (between 14 and 27 US fluid ounces) of bile is formed per day in adult humans.
What are the functions of bile?
- Bile serves as a surfactant to aid in the emulsification of the lipids in food. Bile salt anions have a hydrophilic side and a hydrophobic side, which lead to the aggregation around droplets of lipids (triglycerides and phospholipids) to produce micelles. The micelles' hydrophobic sides face towards the fat, whereas its hydrophilic sides face outwards.
- Since the hydrophilic sides are negatively charged, it prevents bile-coated fat droplets from re-aggregating into larger fat particles. Dickinson & Leser (2007) measured the micelles in the human duodenum typically have a diameter between 1 and 50 μm.
- Food fat break down into micelles to increase the surface area for the enzyme pancreatic lipase to act upon. Mark Lowe (2002) found this allows the pancreatic lipase to digest the triglycerides and approach the fatty core through openings through the bile salts.
- A triglyceride disintegrates into 2 fatty acids and a monoglyceride, which become absorbed by the villi on the intestinal walls. After transporting across the intestinal membrane, the fatty acids re-esterify into triglycerides, before they absorb into the lymphatic system through lacteals.
- A 2016 study found that bile plays an essential role in the absorption of fat-soluble substances, such as vitamins A, D, E and K. Bile functions as a path of excretion for a byproduct of red blood cells recycled by the liver called bilirubin.
- The pH of common duct bile is between 7.5 and 8.05, which is than the pH of the corresponding gallbladder bile (6.80 - 7.65). D. June Sutor (1976) found bile in the gallbladder increases in acidity the longer a human goes without eating, though this increase is reduced when resting.
- Since bile can be slightly alkaline, it can neutralise excess stomach acid before it enters the duodenum. Merritt & Donaldson (2009) found that bile salts can also kill numerous microbes and bacteria that may exist in the consumed food.
ii. Gallbladder
Structure of the gallbladder
- Also known as the cholecyst, the gallbladder is a small hollow organ where bile is stored and concentrated prior to being released into the small intestine. It is situated in a shallow depression under the liver's right lobe.
- In adult humans, the gallbladder is roughly between 7 and 8 cm (between 2.8 and 3.9 inches) long and 4 cm (1.6 in) wide when fully inflated. Its internal capacity is about 50 mm (1.8 ounces).
- It is shaped like a pear that opens into the cystic duct at its tip. It consists of 2 main sections: fundus, body, and neck.
- The fundus has a round base that is angled in order to face the abdominal wall. The body of the gallbladder is located in a depression in the lower surface of the liver. The neck narrows and is continuous with the cystic duct, which is part of the biliary tree.
- The fundus and body of the gallbladder lies against the gallbladder fossa, which is located under the junction of hepatic segments IVB and V. The cystic duct connects with the common hepatic duct to become the common bile duct.
- There is an out-punching of the wall forming a mucosal fold at the junction of the cystic duct and the gallbladder's neck, called the Hartmann's pouch.
- Lymph is drained from the gallbladder through the cystic node, which lies between the cystic duct and the common hepatic duct. In addition, lymphatics from the lower aspect of the gallbladder drain into lower hepatic lymph nodes. All the lymph ultimately drains into the coeliac lymph nodes.
Microanatomy of the gallbladder
The wall gallbladder is composed of multiple layers:
- The innermost surface is lined by a single layer of columnar cells with a brush border of microvilli, which are similar to intestinal absorptive cells.
- Beneath the epithelium is the lamina propria, a muscular layer, an outer perimuscular layer and serosa. One notable observation of the gallbladder is the lack of muscularis mucosae, and the arrangement of muscular fibres in indistinct layers.
- The inner layer of the gallbladder wall is called the mucosa, which contains a lining of a single layer of columnar cells, as well as cells containing minute hair-like attachments called microvilli. It is situated on a thin layer of connective tissue called the lamina propria. The mucosa is curved and assembled into small outpouchings called rugae.
- Underneath the mucosa is a muscular layer composed of smooth muscle, as well as muscle fibres running longitudinally, obliquely and transversely, though not arranged in distinct layers. The muscle fibres contract to excrete bile from the gallbladder.
- There are deep outpounchings of the mucosa called Rokaitansky-Aschoff sinuses that stretch the muscular layer, which is a sign of adenomyomatosis. A layer of connective and fat tissue surrounds the muscular layer, as well as surfaces touching the liver.
- A layer of thick serosa covers the outer layer of the fundus of the gallbladder, as well as the surfaces not touching the liver. It is exposed to the peritoneum, since it contains blood vessels and lymphatics.
Variations of the gallbladder
- It is thought that gallbladder varies in shape, size, and position among every human. A rare phenomenon is a human having more than 1 gallbladder, either as individual bladders draining into the cystic duct, or sharing the same branch that drains into the cystic duct.
- Other abnormalities include gallbladders with 2 lobes separated by a septum, and lack of a gallbaldder. Nonetheless, Leeuw et al. (1995) found these aberrations are unlikely to affect usual function and tend to be asymptomatic.
- The gallbladder may lie in a different location relative to the liver, such as behind, on the left side, within, an separated and dangled from the liver.
- Another anatomical variation of the gallbladder is an innocuous fold in the fundus called a Phrygian cap, which is named after its resemblance to Phrygian cap.
Describe the development of the gallbladder
- Early in development, the human embryo contains 3 germ layers and adjoins an embryonic yolk sac. During week 2 of embryogenesis, the embryo expands and starts to surround and envelop sections of the yolk sac.
- The enveloped sections form the basis for the adult gastrointestinal tract, with parts of this foregut starting to differentiate into the organs of gastrointestinal tract. Gary C. Schoenwolf et al. (2009) described segments of the foregut start to differentiate into the organs of the gastrointestinal tract such as the oesophagus, stomach, and the intestines.
- The stomach begins to rotate during week 4 of embryological development in order for its body to be on the anatomical left side. This rotation consequently affects every part of the gastrointestinal tube below the stomach, which will become the duodenum.
- By the end of week 4, the developing duodenum starts to spurt a small outpouching on its right side called the hepatic diverticulum. This outpouching continues to become the biliary tree.
- A second outpouching appears below the biliary tree called the cystic diverticulum, which eventually develops into the gallbladder.
Describe the functions of the gallbladder
- The principal functions of the gallbladder include storage and concentration of bile (gall), which plays an important role in the digestion of fats in food.
- Bile from the liver flows through small vessels into the larger hepatic ducts and then through the cystic duct into the gallbladder, which it is stored. At any time, between 30 and 60 mm (between 1.0 and 2.0 US fl oz) of bile is stored within the gallbladde.
- When food enters the digestive tract, the fat inside triggers the release of cholecystokinin (CCK) from I cells of the duodenum and jejunum. In response, the gallbladder rhythmically contracts and secretes bile into the common bile duct, eventually draining into the duodenum. The bile emulsifies fats in partially digested foo, thereby aiding their absorption.
- Note that the bile secreted by the liver and stored in the gallbladder is not the same as the bile secreted by the gallbladder. During storage, some water and electrolytes are removed from the bile, increasing its concentration by a factor of 3-10. This occurs through the active transport of sodium and chloride ions across the epithelium of the gallbladder, which produces an osmotic pressure that results in reabsorption of water and other electrolytes.
- The gallbladder can protect against carcinogenesis due to the risk of cancer increasing after cholecystectomy (gallbladder removal). A systematic review and meta analysis by Mu et al. (2023) found that cholecystetomy increases the risk of right-sided colon cancer.
14. Pancreas
- In vertebrates, the pancreas is a heterocrine organ of the digestive exocrine system and endocrine system. Studies conclude that 99% of the pancreas performs exocrine functions, therefore 1% performs endocrine functions.
- The pancreas was first observed by a Greek anatomist and surgeon called Herophilus (335 - 280 BC). A couple of hundred years layer, another Greek anatomist called Rufus of Ephesus first coined the name pancreas to label this organ.
- The word term "pancreas" is a modern Latin adaptation of the Greek word πάγκρεας meaning "all", "whole" (πᾶν) and κρέας ("flesh")], which was originally defined as 'sweetbread'.
- In 1889, Oskar Minkowski removed the pancreas from a dog and discovered this resulted in a diabetes diagnosis. In 1921, Frederick Banting and Charles Best isolated insulin from islets cells of the pancreas.
Development of the pancreas
- The pancreas originates from the foregut, which is a precursor tube to a section of the digestive tract, as a dorsal and ventral bud. During development, the ventral bud rotates to the other side and the 2 buds subsequently fuse together.
- The pancreas develops from the dorsal and ventral buds emerging from the duodenal section of the foregut. The dorsal bud forms the body, neck and tail of the pancreas, whereas the ventral bud forms the head and uncineate process.
- The pancreas fully develops after the ventral bud rotates and the dorsal and ventral buds fuse together. The duodenum, along with the ventral bud, rotates to the right, which shifts it to a more dorsal position.
- When it approaches its final destination, the ventral bud is positioned under the dorsal bud, and eventually fuses with it. This fusion event leads to the formation of the main pancreatic duct.
Cellular development
- Pancreatic progenitor cells differentiate into the functional pancreatic cells, such as endocrine islet cells, exocrine acinar cells, and ductal cells. Carlson (2019) found these precursor cells cc-co-express the transcription factors PDX1 and NKX6-1.
- Differentiation of the exocrine pancreatic cells occur through molecules that trigger differentiation such as fibroblast growth factors, follistatin, and the Notch receptor system.
- The exocrine acini develops through 3 consecutive stages: predifferentiated, protodifferentiated, and differentiated. In addition, they correspond to undetectable, low, and high levels of digestive enzyme activity, respectively.
- The existence of neurogenin-3 and ISL1 stimulates the differentiation of pancreatic progrenitor cells into endocrine islet cells, but only when there is no Notch receptor signalling.
- Under the instruction of a Pax gene, the endocrine precursor cells differentiate to create α and γ cells. Furthermore, under the instruction of Pax-6, the endocrine precursor cells differentiate to create β and δ cells.
- During the 3rd month of development, the endocrine cells migrate from the duct system to produce small clusters around the capillaries called the pancreatic islets. By the 4th or 5th month of development, insulin and glucagon are present in the human foetal circulation.
Structure of the pancreas
- The pancreas is located in the abdomen, extending from behind the stomach to the left upper abdomen adjacent to the spleen. In adults, the pancreas is about 12 - 15 cm (4.7 - 5.9 in) long, as well as divided into lobules.
- The pancreas has 4 anatomical sections: head, neck, body and tail. The head of the pancreas extends from the inner curvature of the duodenum, where it surrounds the superior mesenteric artery and superior mesenteric vein. The body of the pancreas extends from behind the stomach, whereas the tail of the pancreas terminates near the spleen.
- The main pancreatic duct and an accessory pancreatic duct goes through the body of the pancreas. The main pancreatic duct then links with the common bile duct to create a small swelling called the ampulla of Vater (or hepatopancreatic ampulla). Surrounded by a muscle called the sphincter of Oddi, the ampulla of Vater opens into the descending section of the duodenum.
- The sphincter of Boyden manipulates the opening of the common bile duct into the main pancreatic duct. The accessory pancreatic duct connects to the duodenum with separate orifices situated above the opening of the main pancreatic duct.
Describe the sections of the pancreas
- The head of the pancreas is located within the curvature of the duodenum, and swathes around the superior mesenteric artery and superior mesenteric vein. The descending section of the duodenum is situated to the right of the head of the pancreas, and the superior and inferior pancreaticoduodenal arteries runs between them.
- The inferior vena cava and the common bile duct are both located behind the head of the pancreas. The peritoneal membrane and the transverse colon are both situated in front of the head of the pancreas.
- A small uncinate process appears underneath the head of the pancreas, which is located behind the superior mesenteric vein and (occasionally) superior mesenteric artery.
ii. Neck
- The neck of the pancreas is situated in between the head and the body, which is located in the curvature of the duodenum. The neck is about 2 cm (0.79 in) wide, and lies in front of the point where the portal vein emerges.
- It is located mainly behind the pylorus of the stomach, and is enveloped in peritoneum. The anterior superior pancreaticoduodenal arteral runs in front of the neck of the pancreas.
iii. Body
- The body is the largest section of the pancreas, which mainly situates behind the stomach. The peritoneum lies over the body of the pancreas, and the transverse colon lies in front of the peritoneum.
- A number of blood vessels runs behind the body of the pancreas, such as the aorta, the left renal vein, the splenic vein and the start of the superior mesenteric artery.
- Under the body of the pancreas lies the last section of the duodenum and its link to the jejunum, as well as the suspensory ligament of the duodenum which situates between them. The transverse colon lies in front of the body of the pancreas.
iv. Tail
- The pancreas tapers towards the tail, which lies adjacent to the spleen. The tail of the pancreas is between 1.3 and 3.5 cm (between 0.51 and 1.38 in) long, and lies between the layers of the ligament between the spleen and the left kidney. The splenic artery and vein runs behind the tail of the pancreas.
Blood supply of the pancreas
- The pancreas receives blood from the vessels branching off from both the coeliac artery and superior mesenteric artery. The splenic artery branches from the coeliac trunk and stretches along the top of the pancreas, and provides blood to the tail of the pancreas through its pancreatic branches.
- The superior and inferior pancreaticoduodenal arteries travel along the back and front surfaces of the head of the pancreas near the duodenum, which then anastamose in the middle and supply blood to the head of the pancreas.
- The body and neck of the pancreas drain into the splenic vein, which is located behind the pancreas. The head of the pancreas drains into, and swathes, the superior mesenteric and portal veins, via the pancreaticoduodenal veins.
- The pancreas drains into the lymphatic vessels that run alongside the arteries. The lymphatic vessels of the body and tail of the pancreas drain into the splenic lymph nodes, and eventually into the lymph nodes that are located in front of the aorta, between the coeliac and superior mesenteric arteries.
- The lymphatic vessels of the head and neck of the pancreas drain into intermediate lymphatic vessels around the pancreaticoduodenal, mesenteric and hepatic arteries. From there, lymph travels into the lymph nodes located in front of the aorta.
Microanatomy of the pancreas
- A pancreatic islet uses fluorescent antibodies to indicate the location of different cell types. Antibodies against glucagon (released by alpha cells) indicate their peripheral position. Antibodies against insulin (released by beta cells) indicate its more ubiquitous and central position.
- Pancreatic cells form acini around small ducts, which are arranged into lobes with thin fibrous walls. The cells of each acinus produce inactive digestive enzymes called zymogens into the small intercalated ducts.
- Each acinus contains pyramid-shaped cells that are located around the intercalated ducts, with the nuclei lying on the basement membrane, a large endoplasmic reticulum, and a number of zymogen granules visible within the cytoplasm.
- The intercalated ducts drain into larger intralobular ducts within the lobule, and ultimately interlobular ducts. The ducts are usually lined by a single layer of column-shaped cells, which increase by layer as the diameter of the duct increases.
- The tissues within the pancreas that exist as clusters of cells and have endocrine functions are called pancreatic islets (islets of Langerhans). Pancreatic islets consist of alpha (α) cells, beta (β) cells, and delta (δ) cells, each of which produce a different hormone.
- α cells produce glucagon and are located around the periphery of the islet, whereas β cells produce insulin and found throughout the islet. In addition, enterochromaffin cells are also situated throughout the islet.
- Each pancreatic islet consists of up to 3,000 secretory cells, as well as several small arterioles to attain blood, and venules for hormones secreted by the cells to enter the systemic circulation.
Variations of the pancreas
- In about 10% of adults, an accessory pancreatic duct may emerge if the main duct of the dorsal bud of the pancreas fails to regress, which opens into the minor duodenal papilla.
- If the dorsal and ventral buds don't fuse, a pancreas with 2 separate ducts will emerge, a condition known as a pancreas divisum.
- If the ventral bud doesn't fully rotate, this may result in an annular pancreas, where a portion or all of the duodenum is surrounded by the pancreas. Noh et al. (2012) thought this condition may be associated with duodenal atresia.
Gene and protein expression
- Approximately 10,000 protein coding genes (~50% of all human genes) are expressed in the human pancreas, with less than 100 of these genes expressed specifically in the pancreas.
- Pancreas-specific proteins expressed in the exocrine cellular compartment elicit functions associated with digestion or food uptake. Examples of such proteins include digestive chymotrypsinogen enzymes and pancreatic lipase PNLIP.
- Pancreas-specific proteins expressed in the various cells of the various pancreatic islets elicit functions associated with secreted hormones. Examples include glucagon, insulin, pancreatic polypeptide, and somatostatin.
What are the functions of the pancreas?
i. Blood glucose regulation
- When blood glucose levels are low, alpha cells release glucagon in order to increase blood glucose levels. When blood glucose levels are high, beta cells release insulin to decrease blood glucose levels. This means the pancreatic cells are actively maintaining blood glucose levels as part of homeostasis. Delta cells in the pancreatic islet also release somatostatin that reduces the production of insulin and glucagon.
- Glucagon increases glucose levels by stimulating the production of glucose and the breakdown of glycogen to glucose in the liver. In addition, glucagon reduces the uptake of glucose in fat and muscle.
- Glucagon production is triggered by low blood glucose or insulin levels, as well as during exercise. In contrast, insulin reduces blood glucose levels by facilitating uptake by cells (especially skeletal muscle), and augmenting its usefulness in the production of carbohydrates, fats and proteins.
- Insulin is first produced as a precursor form called preproinsulin, which is then converted to proinsulin and cleaved by C-peptide to insulin. This newly created insulin is subsequently stored in granules in beta cells. Glucose is received by the beta cells and then degraded by those cells. This results in depolarisation of the cell membrane, therefore triggers the release of insulin.
- Insulin and glucagon release can be triggered by the presence of amino acids, which are byproducts of protein digestion. On the other hand, somatostatin acts to inhibit the function of insulin and glucagon.
- Kim Barrett (2019) found activation of β2 receptors of the sympathetic nervous system by catecholamines released by sympathetic nerves triggers the release of insulin and glucagon, whereas activation of the α1 receptors inhibits release of insulin and glucagon.
- When the right vagus nerve stimulates M3 receptors of the parasympathetic nervous system, it triggers secretion of insulin from β cells.
ii. Digestion
- The pancreas releases a fluid that contains digestive enzymes into the duodenum, which break down carbohydrates, lipids and proteins. This is the exocrine stage of the pancreas.
- About 1.5 - 3 L of fluid are released into the middle of the acinus where it accumulates in intralobular ducts. The fluid subsequently drains into the main pancreatic duct, which drains directly into the duodenum.
- The cells in each acinus are filled with granules that contain the digestive enzymes, which are secreted in an inactive form called zymogens or proenzymes. Harrison's 2015 study found the zymogens released into the duodenum are activated by the enzyme enterokinase existing in the lining of the duodenum. The proenzymes are cleaved, which result in a cascade of activating enzymes. Examples include:
- Proteases initiated by the activation of trypsinogen to trypsin, which subsequently cleaves the rest of the trypsinogen, as well as chymotrypsinogen to its active form chymotrypsin.
- Lipases such as phospholipase A2, lysophospholipase, and cholesterol esterase
- Amylase that break down starch and carbohydrates
- These enzymes are released in an alkaline fluid full of bicarbonate, because it maximises the efficiency of the enzymes, as well as neutralises the stomach acids that enter the duodenum. This release of the fluid is regulated by hormones such as cholecystokinin, secretin, and VIP, as well as acetylcholine stimulation from the vagus nerve.
- Secretin is secreted from the S cells located in the lining of the duodenum in response to stimulation by gastric acid. In addition, secretin and VIP increases the release of enzymes and bicarbonate.
- Cholecystokinin is secreted by Ito cells located in the lining of the duodenum and jejunum mainly in response to long chain fatty acids, which enhances the effects of secretin.
- Bicarbonate is released by centroacinar and ductal cells through a sodium and bicarbonate co-transporter due to membrane depolarisation triggered by the cystic fibrosis transmembrane conductance regulator. Studies found both secretin and VIP increase the opening of the cystic fibrosis transmembrane conductance regulator, which results in further membrane depolarisation and furtehr secretion of bicarbonate.
- To ensure the digestive action of the pancreas doesn't digest pancreatic tissue itself, a number of inactive enzymes (zymogens) and the protective enzyme trypsin inhibitor are secreted to inactivate trypsin. Furthermore, the pH of the fluid increases with bicarbonate secretion upon activation of the pancreas, as well as calcium level reduction within cells leading to trypsin inactivation also play important roles in protecting the pancreatic itself from self-digestion.
C. Intestinal phase
This stage begins in the duodenum, where the partially digested food is combined with a number of enzymes produced by the pancreas to be disintegrated even further.
15. Small Intestine
- Known as a small bowel, the small intestine is an organ in the gastrointestinal tract where a majority of the absorption of nutrients from food occurs.
- The small intestine develops from the midgut of the primitive gut tube. By week 5 of embryological development, the ileum starts to lengthen at a rapid rate, creating a U-shaped fold called the primary intestinal loop. The growth of the loop is faster than the abdomen's rate of growth, which results in its protrusion through the umbilicus. By week 10, the loop then retracts back into the abdomen.
- Between weeks 6 and 10, the small intestine rotates counter-clockwise, as seen from the front of embryo. Then it rotates an additional 180 degrees after it has returned into the abdomen. This results in the twisted shape of the large intestine.
Structure of the small intestine
a. Size
- The length of the small intestine varies from as short as 3 metres (10 feet) to as long as 10.5 m (34.5 feet). Factors include the height of the person and method used to measure the length. DiBaise et al. (2016) found taller people tend to have longer small intestines and measurements become longer after death and when the bowel is empty.
- It is roughly 1.5 cm (5/8 inch) wide in newborns after 35 weeks of gestational age. This grows to 2.5 - 3 cm (1 - 9/8 inches) wide in adults. On abdominal X-rays and CT scans, if the diameter of the small intestine is bigger than 3 cm, then it is regarded as abnormally dilated. Due to enlargement caused by folds, the average surface area of the human small intestinal mucosa is 30 square metres (320 sq ft).
b. What are the small parts of the small intestine?
i. Duodenum
- In a majority of vertebrates, such as mammals, reptiles, and birds, the duodenum is the first segment of the small intestine. In humans, the duodenum is a C-shaped hollow jointed tube that is 25 - 38 cm (10 - 15 in) long, located adjacent to the stomach (and linking it to the small intestine).
- The name duodenum is derived from a Medieval Latin phrase, short for intestīnum duodēnum digitōrum, meaning intestine of twelve (12) finger-widths (in length), genitive pl. of duodēnī, twelve (12) each, from Latin duodeni "twelve (12) each" (from duodecim "twelve"(12)).
- It was coined by Gerard of Cremona in his 12th century Latin translation of "Canon Avicennae," "اثنا عشر" itself a loan-translation of Greek dodekadaktylon, literally "twelve fingers long." The Greek physician Herophilus (c. 335 - 280 BCE) described the length of the intestine section to be equal to about the breadth of 12 fingers.
Describe the different parts of the duodenum
- The 1st or superior section of the duodenum is a continuation from the pylorus to the transpyloric plane. It is superior to the other segments at L1 vertebral level. The 1st section of the duodenum includes the duodenal bulb, which is about 2 cm (3/4 in) in length and is slightly dilated.
- The duodenal bulb is a remnant of the mesoduodenum, which is a mesentery that hangs the organ from the posterior abdominal wall in foetal life. This section is mobile, and is linked to the liver by the hepatoduodenal ligament of the lesser omentum, ending at the corner known as the superior duodenal flexure.
- The 2nd (or descending) section starts at the superior duodenal flexure. It then runs inferior to the lower border or vertebral body L3, before it turns medially into the inferior duodenal flexure, where the descending section ends.
- The pancreatic duct and common bile duct enter the descending duodenum via the major duodenal papilla. The second section of the duodenum also contains an entrance for the accessory pancreatic duct at the minor papilla. The junction between the embryological foregut and midgut is situated under the major duodenal papilla.
- The 3rd (transverse or horizontal or inferior) section of the duodenum starts at the inferior duodenal flexure and runs transversely to the left, then in front of the inferior vena cava, abdominal aorta and the vertebral column. This part is usually between 10 and 12 cm long.
- Lying anteriorly to the 3rd section of the duodenum are the superior mesenteric artery and vein. If this section is compressed between the aorta and superior mesentery artery, this lead to superior mesenteric artery syndrome.
- The 4th (ascending) section of the duodenum runs upward, linking with the jejunum at the duodenojejunal flexure. It is located at the vertebral level L3, and may run directly on top, or slightly to the left, of the aorta.
Blood supply of the duodenum
- Proximal to the 2nd section of the duodenum (around the major duodenal papilla), the arterial supply comes from the gastroduodenal artery and its branch the superior pancreaticoduodenal artery.
- Distal to the midgut, the arterial supply comes from the superior mesentery artery, and its branch the inferior pancreaticoduodenal artery supplies the 3rd and 4th sections.
- The superior and inferior pancreaticoduodenal arteries (from the gastroduodenal artery and the superior mesenteric artery respectively) create an anastomotic loop between the coeliac trunk and the superior mesentery artery, thus a potential for collateral circulation.
- The veins from the duodenum drain into the portal system, either directly or indirectly through the splenic or superior mesenteric vein and ultimately to the portal vein.
Lymphatics of the duodenum
- The anterior lymphatic vessels drain into the pancreatoduodenal lymph nodes situated along the superior and inferior pancreatoduodenal arteries and subsequently into the pyloric lymph nodes (along the gastroduodenal artery).
- The posterior lymphatic vessels run posterior to the head of the pancreas and drain into the superior mesenteric lymph nodes. Efferent lymphatic vessels originating from the duodenal lymph nodes eventually run into the coeliac lymph nodes.
Histology of the duodenum
- Under a microscope, the duodenum contains a villous mucosa, which is different from the mucosa of the pylorus. It contains a mucosa, submucosa, muscularis externa, and adventitia.
- The glands lining the duodenum are called Brunner's glands, which produce mucus and bicarbonate to neutralise stomach acids.
Variations of the duodenum
- A congenital abnormality called the annular pancreas results in a section of the pancreas to encompass the duodenum. In an extramural annular pancreas, the pancreatic duct encompasses the duodenum that results in gastrointestinal obstruction. Borghei et a. (2013) found an intramural annular pancreas is characterised by pancreatic tissue that is fused with the duodenal wall, resulting in duodenal ulceration.
- These variations indicate the duodenum's close anatomical association with the pancreas results in differences in function based on the position and orientation of the organs.
Gene and protein expression
- About 20,000 protein coding genes are expressed in human cells and about 70% of them are expressed in the normal duodenum. About 300 of these genes are more specifically expressed in the duodenum with a small number of genes expressed only in the duodenum.
- The corresponding specific proteins are expressed in the duodenal mucosa, and a majority of these genes are also expressed in the small intestine. Examples of proteins such as alanine aminopeptidase, angiotensin-converting enzyme and RBP2, which play roles in digestion, regulation of blood pressure, and uptake of vitamin A.
What are the functions of the duodenum?
- The duodenum plays a major role in the breakdown of food in the small intestine with the aid of enzymes.
- The duodenum is involved in the regulation of the emptying of stomach's contents via hormonal pathways. Secretin and cholecystokinin are secreted from cells in the duodenal epithelium in response to acidic and fatty substances when the pylorus opens and releases gastric chyme into the duodenum for additional digestion.
- Ducts from the pancreas and gallbladder deliver bicarbonate, pancreatic enzymes, and bile salts at the major duodenal papilla in the descending duodenum. This results in neutralisation of acid in gastric secretions, additional digestion, and emulsification of fat, respectively.
- Brunner glands release mucus only into the superior segment of the duodenum in order to lubricate and protect the mucosal layer of the small intestine.
ii. Jejunum
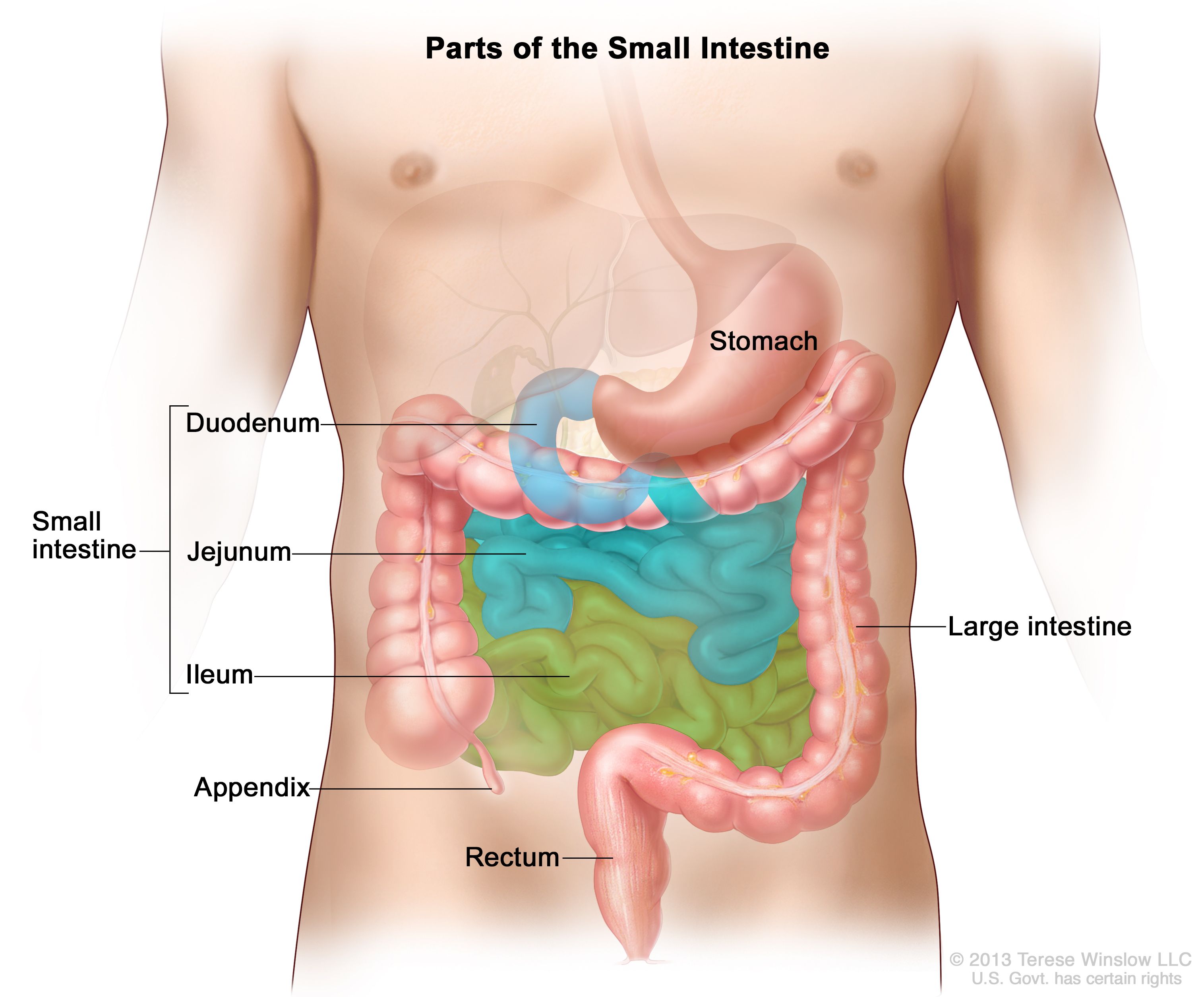
- The jejunum is the 2nd section of the small intestine in humans and a majority of higher vertebrates, including birds, mammals and reptiles. Its lining is specialised for the absorption by enterocytes of microscopic nutrient molecules that were digested by enzymes in the duodenum.
- Since the jejunum is situated between the duodenum and the lieum, it is thought to begin at the suspensory muscle of the duodenum, located at the duodenojejunal flexure. The jejunum is estimated to be about 40% of the length of the small intestine, which is about 2.5 m (8.2 ft).
- The word jejunum originates from the Latin word jējūnus (iēiūnus), meaning "fasting." It is called jejunum because it is often observed to be void of food post-mortem, due to the peristaltic activity relative to the duodenum and ileum.
- The inner surface of the jejunum contains a layer of finger-like projections of mucosa called villi, which increase the surface area of tissue to absorb nutrients from ingested food. The epithelial cells lining the villi contain microvilli.
- There is passive transport of sugar fructose and active transport of amino acids, small peptides, glucose and vitamins across the epithelial cells through the jejunum and ileum. The jejunum and the ileum are connected by mesentery, which provide the bowel mobility within the abdomen. In addition, the jejunum contains circular and longitudinal smooth muscle that aid in transporting food down the small intestine by peristalsis.
- The pH in the jejunum is between 7 and 8, which is regarded as neutral or slightly alkaline.
Histology of the jejunum
- The jejunum contains a small number of Brunner's glands or Peyer's patches. However, there are some jejunal lymph nodes situated in its mesentery. The jejunum contains numerous large circular folds in its submucosa called plicae circulares that increase the surface area for nutrient absorption.
- Although there is no line of demarcation between the jejunum and the ileum, there are histological differences between them.
- The ileum has more fat inside its mesentery than the jejunum.
- The ileum has a smaller diameter than the jejunum.
- There are long, finger-like projections called villi in the jejunum.
- While the whole length of the intestinal tract contains lymphoid tissue, only the ileum contains abundant Peyer's patches, which are exposed lymphoid nodules that consist of numerous lymphocytes and immune cells.
What are the functions of the jejunum?
The lining of the jejunum is specialised for the absorption by enterocytes of of minute nutrient molecules that were previously digested by enzymes in the duodenum. After the nutrients are absorbed (except for fat), they move from the enterocytes into the enterohepatic circulation and enter the liver via the hepatic portal vein, where blood is processed.
iii. Ileum
- The ileum is the 3rd segment of the small intestine in a majority of higher vertebrates, including birds, mammals, and reptiles. The main function of the ileum is absorption of vitamin B12, bile salts, and other products of digestion that were not absorbed by the jejunum.
- The word ileum originates from the Greek word εἰλεός (eileós), referring to a medical condition known as ileus.
Development of the ileum
- By week 5 of embryological development, the ileum starts to lengthen at a rapid rate, creating a U-shaped fold called the primary intestinal loop. The proximal half of the primary intestinal loop will form the ileum.
- The loop's rapid growth outpaces the growth of the abdomen, which makes it stick our through the umbilicus. Between weeks 6 and 10, the small intestine rotates anticlockwise, as viewed from the front of the embryo. By week 10, the loop U-turns into the abdomen. Then, it rotates a further 180 degrees after shifting back into the abdomen, which results in the twisted shape of the large intestine.
- In the foetus, the ileum links with the navel by the vitelline duct. In about the 2-4% of humans, the vitelline duct doesn't close during the first 7 weeks after birth, which results in Meckel's diveticulum.
Structure of the ileum
- The ileum is connected to the jejunum and is separated from the caecum by the ileocecal valve (ICV). In humans, the ileum is between 2 and 4 metres long, and the pH is typically between 7 and 8.
- Like the duodenum and the jejunum, the ileum is situated inside the mesentery, which is a peritoneal structure that carries the blood vessels supplying them (i.e. the superior mesenteric artery and vein).
Histology of the ileum
There are 4 layers in the wall of the ileum, which are consistent with those of the gastrointestinal tract. From the inner surface to the outer surface, these layers are:
- A mucous membrane formed by 3 sublayers:
- A single layer of large cells lining the lumen of the organ. The epithelium forms the innermost section of the mucosa, which contains 5 distinct types of cells: enterocytes with microvilli, goblet cells, Paneth cells, microfold cells and enteroendocrine cells.
- Each type of cell elicits its own function: enterocytes digest and absorb nutrients; goblet cells produce mucin to lubricate the wall of the organ; Paneth cells are only located at the bottom of the intestinal glands and produce anticrobial substances such as α-defensins and lysozyme; microfold cells capture and transport antigens from the lumen to lymphatic cells of the lamina propria; enteroendocrine cells produce hormones.
- An underlying lamina propria consisting of connective tissue, as well as germinal centres and large aggregates of lymphoid tissue called Peyer's patches.
- A thin layer of smooth muscle called the muscularis mucosae.
- A submucosa composed of dense irregular connective tissue that supports the large blood vessels and the submucosal plexus, which is a component of the enteric nervous system.
- An external muscular layer consisting of 2 layers of smooth muscle arranged in circular bundles in the inner layer and in longitudinal bundles in the outer layer. The myenteric plexus lies in between the 2 smooth muscle layers, which is also part of the enteric nervous system.
- A serosa made of mesothelium, which is a single layer of flat cells with various amounts of connective and adipose tissue. This layer contains the visceral peritoneum and is continuous with the mesentery.
Functions of the ileum
- The principal function of the ileum is to absorb products of digestion not absorbed by the jejunum such as bile salts and vitamin B12. The ileum wall is composed of folds, each of which contains numerous villi on its surface. Furthermore, the epithelial cells lining these villi contain even more numerous amounts of microvilli.
- As a result, the ileum has a tremendously larger surface area both for the adsorption of enzyme molecules and for the absorption of products of digestion.
- The cells of the diffuse neuroendocrine system (DNES) produce various hormones into the bloodstream, such as cholecystokinin, secretin, and gastrin. The lining of the ileum contains cells that produce protease and carbohydrase enzymes that play important roles in the final stages of protein and carbohydrate digestion into the lumen of the intestine.
- The villi contains numerous capillaries that transport the amino acids and glucose yielded by digestion to the hepatic portal vein and the liver. The small lymph vessels in villi are called lacteals, which absorb the products of fat digestion such as fatty acids and glycerol.
- The layers of circular and longitudinal smooth muscle allow the chyme to be propelled along the ileum by peristalsis. The remaining chyme moves on to the colon.
16. Large Intestine
Describe the structure of the large intestine
- Known as the large bowel, the large intestine is the final section of the gastrointestinal tract and of the digestive system in tetrapods and mammals. It appears segmented because it consists of a series of saccules called haustra.
- The colon is the longest section of the large intestine and it is about 166 cm (65 in) long (80 - 313 cm range) in the adult males, and about 155 cm (61 in) long (80 - 214 cm) in the adult females.
- In mammals, the large intestine is composed of the caecum (including the appendix), colon, rectum, and anal canal. The 4 main sections of the colon are: the ascending colon, transverse colon, descending colon, and sigmoid colon.
- The ascending colon, descending colon and rectum are retroperitoneal, meaning they are partially covered in peritoneum. On the other hand, the appendex, caecum, transverse colon and sigmoid colon are intraperitoneal, meaning they are completed covered in peritoneum and are thus mobile.
- The caecum has the largest diameter with an average of about 9 cm in healthy individuals, and the transverse colon has the second largest diameter with an average of about 6 cm. The descending and sigmoid colon are slightly narrower, with the sigmoid colon having an average diameter of 4 - 5 cm (1.6 - 2.0 in).
a. Caecum
- The caecum (cecum) is a pouch within the peritoneum marking the start of the large intestine. It is usually located on the anatomical right side of the body.
- The term caecum is derived from Latin (intestinum) caecum meaning "blind intestine', in the sense 'blind gut' or 'cul de sac'. It is directly translated from Ancient Greek τυφλὸν (ἔντερον) typhlòn (énteron). Hence, the inflammation of the caecum is called typhlitis.
- The junction between the small intestine and the colon is called the ileocecal valve, where it is the end point for the colon with a dead-end section ending with the appendix. The link between the caecum and the ascending colon is called the cecocolic orifice.
Development of the caecum
- The caecum and appendix originate from the bud of the caecum that emerge during week 6 in the midgut next to the apex of the umbilical herniation. The caecum and appendix are specifically formed by the enlargement of the postarterial section of the midgut loop.
- The proximal segment of the bud expands quickly to create the caecum. Kostouros et al. (2020) discovered the position of the caecum alters after midgut rotates and the ascending colon lengthens, and the diameter of ascending colon may increase due to accumulation of meconium inside the caecum.
Functions of the caecum
- The caecum receives chyme from the ileum, and joins the ascending colon of the large intestine. Sometimes referred to as Bauhin's valve, the ileocecal valve (ICV) separates the caecum from the ileum. In addition, the cecocolic junction separates the caecum from the colon. The caecum is typically intraperitoneal, whereas the ascending colon is retroperitoneal.
- In herbivores, the caecum stores food substance where bacteria can break down the cellulose. In humans, the caecum plays important roles in absorption of salts and electrolytes, and lubrication of solid waste that moves into the large intestine.
b. Appendix
- The appendix (plural: appendices or appendixes) is a finger-like, deadend tube linked to the caecum, from which develops in the embryo.
- Charles Darwin thought the primary function of the appendix in earlier hominids was digestion of fibrous vegetation, which then evolved over time. Some herbivorous animals have long caecums, such as the horse or koala, which might support this hypothesis. Moreover, the koala's caecum is found to host bacteria that aid in breaking down cellulose.
- It is believed human ancestors had similar caecums when they survived on a diet abundant in foliage. Over time, more and more humans consumed more foods that were easily digested, their reliance on plant food rich in cellulose for energy decreased. As the caecum's role in digestion decreased in usefulness, mutations that were previously detrimental were no longer essential, as the mutations survived.
- In 1871, Charles Darwin hypothesised that these alleles increased in frequency and the caecum continued to shrivel. Over millions of years, the caecum gradually shrinked to be the appendix observed in modern humans.
- Smith et al. (2009) explained that the mammalian caecal appendix served as a storage location for symbiotic gut microbes, which preserved the gut flora during instances of gastrointestinal infection in societies that lacked modern medicine. Furthermore, the evolution and maintenance of the appendix was selectively pushed by its main function of preserving gut bacteria.
- Smith et al. (2013) discovered the appendix independently evolved in different animals more than 30 times and have disappeared on no more than 6 times over the couse of history. Smith et al. (2017) discovered similar results using similar methodology on an updated database, which indicated that the caecal appendix has a selective advantage in numerous scenarios and presented a strong argument against its vestigial nature.
- Collard et al. (2021) thought the appendix's selective advantage in many situations correlated with larger maximal longevity. Collard et al. (2023) discovered the young primates demonstrated protective functions against diarrhoea.
- Due to the complex evolutionary history of the appendix, along with the hetereogeneity in its evolutionary rate in different taxa, Laurin, Everett & Walker (2011) concluded that this is a recurrent trait.
- In 2001, the World Health Organisation collected epidemiological data on the cause of death in developing countries. They found that acute diarrhoea was the 4th leading cause of disease-associated death in development countries. A 2009 report by Duke Medicine News and Communications expected 2 of the other leading causes of death to apply limited or no selection pressure.
Structure of the appendix
- The average length of the human appendix is about 9 cm (3.5 in), with a range of 5 - 35 cm (2.0 - 13.8 in). The diameter of the normal appendix is about 6 mm (0.24 in). If it is greater than 6 mm (0.24 in), it indicates it is either thickened or inflamed.
- The appendix typically lies in the lower right quadrant of the abdomen, close to the right hip bone. The base of the appendix lies about 2 cm (0.79 in) under the ileocecal valve that separates the large intestine from the small intestine. The appendix is linked to the mesentery in the lower part of the ileum, by a short section of the mesocolon called the mesoappendix.
Variations of the appendix
- Some identical twins have a mirror-imaged anatomy, a congenital condition with the appendix located in the lower left quadrant of the abdomen rather than in the lower right quadrant. If the intestines rotate the wrong way, this may result in displacement of the appendix to the left side.
- While the base of the appendix usually lies around 2 cm (0.79 in) under the ileocecal valve, the location of the tip of the appendix varies, such as behind the caecum, outside the peritoneum or in the pelvis.
- Studies found about 67.3% of Ghanian and 58.3% of Sudanese people have appendices in the retrocecal position, whereas about 55.8% of Iranian and 57.7% of Bosnian people have appendices in the pelvic position.
- Zetina-Mejía et al. (2009) estimated about 1 in 100,000 people lack an appendix after a laparotomy due to appendicitis.
- Golalipour et al. (2003) described a semi-circular fold of mucous membrane forming at the opening of the appendix. This valve of the vermiform appendix is called Gerlach's valve.
What are the functions of the appendix?
i. Maintenance of gut flora
- In 2007, Parker et al. conjectured that the appendix functions as a refuge for acteria when illness expels the bacteria from the rest of the intestines. This hypothesis is based on the theory of the immune system augmenting the growth of useful intestinal bacteria, and the appendix's location being beneath the unidirectional flow of food in the large intestine, and its link to the immune tissues.
- In 2012, Rob Dunn found that humans lacking an appendix were 4 times as likely to have a recurrence of Clostridium difficile colitis. A 2007 report by NBC News suggested the appendix serves as a "safe house" for useful bacteria.
- Randal Bollinger et al. (2007) found this bacteria reservoir can repopulate the gut flora in the digestive system after a period of cholera or dysentery, or to enhance it after a mild gastrointestinal illness.
ii. Immune and lymphatic systems
- The appendix is determined to play an important role in mammalian mucosal immune function, especially B cell-mediated immune responses and extrathymically derived T cells.
- It aids in the transport and removal of waste matter in the digestive system. Moreover, the appendix contains lymphatic vessels that mediate pathogens, and may play a role in early defences to prevent deadly diseases.
- Zahid (2004) hypothesised that the appendix offers additional immune defences from invading pathogens and primes the B and T cells to combat the pathogens that infect that section of the bowel. The appendix augments the maturation of the immune cells, which results in targeted immune responses and effective and safe elimination of pathogens.
- Rankin et al. (2016) identified innate lymphoid cells act in the gut to support the appendix maintain a human's digestive health. Smith et al. (2016) found a positive correlation between the existence of the appendix and the cluster of caecal lymphoid tissue, which supports the theory that appendix evolves as a complex with the caecum as well as provide major immune benefits.
c. Ascending colon
https://en.wikipedia.org/wiki/Ascending_colon- In humans and homologous primates, the ascending colon is located between the caecum and the transverse colon. In addition, it is located on the right side of the body (except for malformations). In ruminants, it is referred to as the spiral colon.
- The ascending colon runs upward, opposite the colic valve, to the under surface of the right lobe of the liver, where it lies in the colic impression. It then curves sharply forward and to the left, becoming the right colic flexure where it transitions to the transverse colon. The ascending colon remains in contact with the posterior wall of the abdomen by the peritoneum.
- The vagus nerve and the thoracic splanchnic nerves supply parasympathetic and sympathetic nerve stimuli to the ascending colon, respectively.
- The process of separating water and other key nutrients from waste material and subsequently recycling it begins in the ascending colon.The waste material is then forced upwards toward the transverse colon by peristalsis. Studies found the ascending colon occasionally connects to the appendix via Gerlach's valve.
d. Transverse colon
- The transverse colon is the longest and most mobile section of the colon, as it is enveloped in peritoneum.
- It crosses the abdomen from the ascending colon at the right colic flexure (hepatic flexure) with a downward curvature to the descending colon where it bends abruptly on itself under the lower of the spleen to create the left colic flexure (splenic flexure).
- The transverse colon is arched-shaped, with its concave curvature directed backward and slightly upward. There is a sharp U-shaped curve towards its splenic end, which may descend lower than the main curve.
- It is virtually encompassed by the peritoneum, and is linked to the inferior border of the pancreas by a fold of a membrane called the transverse mesocolon. It hangs off the stomach, which is connected by a large fold of peritoneum called the greater omentum.
- A branch of the superior mesenteric artery (SMA) called the middle colic artery provides blood to the proximal 2/3rds of the transverse colon. Furthermore, the branches of the inferior mesenteric artery (IMA) provides blood to the distal 3rd of the transverse colon.
e. Descending colon
- In humans and homologous primates, the descending colon stretches from the left colic flexure to the level of the iliac crest. It is typically located on the anatomical left side of the body (unless there have been malformations). The descending colon is typically retroperitoneal, and may be suspended mesentery in some people.
- The main function of the descending colon is to store waste until it can be eliminated from the body in solid form (i.e. stool) during a bowel movement. The stools slowly solidify as they shift along the descending colon.
f. Sigmoid colon
- Known as the pelvic colon, the sigmoid colon lies between the descending colon and the rectum and anus. The sigmoid colon consists of a loop that is 35 - 40 cm (14 - 16 in) long, which is usually shaped like a the Greek litter sigma (ς) or Latin letter S (thus sigma + -oid). This section of the colon usually lies within the pelvis, and is likely to be displaced into the abdominal cavity due to its motility.
- The sigmoid colon runs from the superior aperture of the lesser pelvis, and passes transversely across the front of the sacrum to the right of the pelvis. Next, it bends on itself and curves toward the left to approach the middle line at the level of the 3rd sacral segment, where it curves downward and terminates in the rectum.
- The sigmoid colon is entirely encompassed by peritoneum, which forms a mesentery (or sigmoid mesocolon). The peritoneum shortens from the centre towards the ends of the loop, where it terminates. This allows the loop to be fixed at its junctions with the iliac colon and rectum.
- The main function of the sigmoid colon is to excrete solid and gaseous waste from the gastrointestinal tract. The curved trajectory towards the anus enables the sigmoid colon to store gas in the superior arched section, which facilitates the colon to release gas without excreting stools simultaneously.
- The pelvic splanchnic nerves and the lumbar splanchnic nerves provide parasympathetic and sympathetic innervation, respectively.
g. Rectum
- The rectum is the last straight section of the large intestine in humans and other mammals, and the gut in other animals. The adult human rectum is on average 12 cm (4.7 in) in length, and starts at the rectosigmoid junction at the S3 vertebral level, or the sacral promontory.
- Although its diameter is close to the diameter of the sigmoid colon at the beginning, it is dilated near the end, creating the rectal ampulla. The rectum ends at the level of the anorectal ring or the dentate line.
- The word rectum originates from the Latin intestinum rectum meaning 'straight gut'. In addition, it is a calque of Ancient Greek ἀπευθυσμένον ἔντερον, derived from ἀπευθύνειν, to make straight and ἔντερον, gut, which is verified by the Greek physician Galen. In 1543, Andreas Vesalius contributed to the knowledge of the rectum by publishing descriptions of its anatomy.
Describe the structure of the rectum
- The rectum is a continuation of the sigmoid colon, and links with the anus. The rectum follows the shape of the sacrum and terminates in the ampulla where faecal matter is stored before its excretion via the anal canal.
- It starts at the S3 vertebral level where it links with the sigmoid colon, and ends as it passes through the plevic floor muscles where it links with the anal canal.
- The recrum lacks distinct taeniae coli, because it blends with one another and ends in the sigmoid colon about 5 cm above the rectum. It becomes a singular longitudinal muscle that surrounds the rectum throughout its length.
Blood supply and venous drainage of the rectum
- The superior rectal artery provides blood to the top 2/3rds of the rectum, whereas the middle and inferior rectal arteries supplies blood to the lower 3rd of the rectum.
- The superior rectal artery is continuous with the inferior mesenteric artery, where it cross the pelvic brim. It then enters the mesorectum at the S3 vertebral level, and subsequently divides into 2 branches, which stretch at the lateral posterior section of the rectum, and lastly the sides of the rectum. These branches then terminate in branches in the submucosa, which anastamose with branches of the middle and inferior rectal arteries.
Microanatomy of the rectum
- The wall of the mucosa contains a single layer of column-shaped cells with interspersing goblet cells, sitting on the lamina propria, and a layer of smooth muscle called the muscularis mucosa.
- The muscularis mucosa lies on an underlying submucosa of connective tissue, which is confined by a muscularis of propria consisting of an inner circular and an outer longitudinal band of muscle.
- In 2013, Wheater found the rectal mucosa contains a greater concentration of goblet cells than other sections of gastrointestinal tract. In addition, the lining of the rectum of alters drastically at the border where the rectum connects to the anus. At this point, the lining alters from column-shaped cells to numerous layers of flat cells.
What are the functions of the rectum?
- The main function of the rectum is to temporarily store faeces, which is provided by the colon, transferred through peristalsis. As more faeces enter the rectum, the rectal walls expands until it triggers stretch receptors to send the signal to the nervous system to pass the faeces, a process known as defecation.
- To prevent faecal incontinence (i.e. leakage of faeces), 2 anal sphincters (internal and external) and resting contraction of the puborectalis muscle are required.
- As the rectum continues to become more bloated, it's a matter of time before the sphincters relax and a reflex excretion of the faecal content from the rectum transpires.
- When the rectal pressure reaches 18 mmHg, this results in an impulse to voluntarily defecate. If the humans doesn't defecate and continues to allow the rectal pressure to increase, they will experience reflex expulsion at 55 mmHg.
- Voluntary defecation occurs when the rectal muscles and abdominal muscle contract, and the puborectalis muscle and the external anal sphincter relaxes. This straightens the angle between the rectum and anus, which facilitates the process of defecation.
h. Anal Canal
- The anal canal joins the rectum with the anus, located under the level of the pelvic diaphragm. Moreover, it lies within the anal triangle of the perineum, between the right and left ischioanal fossa.
- In humans, the anal canal is between 2.5 and 4 cm (0.98 - 1.57 in) in length, from the anorectal junction to the anus. It is surrounded by inner involuntary and outer voluntary sphincters that keep the lumen closed.
Describe the structure of the anal canal
The anal canal is composed of upper and lower segments, which are bisected by the pectinate line or the dentate line.
- The anal verge refers to the distal end of the anal canal, which is a transitional zone between the epithelium of the anal canal and the perianal skin.
- The anal gland releases lymphatic discharge and accumulated faecal matter from the colon lining.
- The ischioanal fossa lie on each side of the anal canal, and the perianal space encompasses the anal canal under the white line called Hilton's line.
- The submucous space of the anal canal is located above Hilton's line between the mucous membrane and internal anal sphincter muscle.
- The external anal sphincter muscle is the voluntary muscle that encompasses and connects to the anus at the lower margin of the anal canal. This muscle usually is tonically contracted, but it relaxes during defecation in order for the excretion of faeces.
- The involuntary internal anal sphincter is an extension of the circular muscle encompassing the anal canal. When the muscle is contracted, it holds up the faeces. When the circular muscle relaxes, the internal anal sphincter also relaxes, which allows the anal canal to discharge the faeces.
How does bacteria break down food?
- Living inside animals' digestive tracts are microorganisms, including archaea, bacteria, fungi, and viruses, known as the gut microbiota, gut microbiome, or gut flora. In humans, the gut microbiota contains the most abundant and most diverse species of bacteria compared to other parts of the body.
- Turnbaugh et al. (2007) estimated about 1*1013 to 1*1014 bacteria compose the gut microbiota. The gut flora is established in humans at birth and gradually matures towards a gut microbiome similar to an adult's by the age of two years. This corresponds with the development and maturation of the intestinal epithelium and intestinal mucosal barrier.
- Sommer & Bäckhed (2013) stated the intestinal mucosal barrier plays a critical role in supporting the a symbiotic relationship with the gut flora whilst offering protection against pathogenic organisms.
- Sherwood, Willey & Woolverton (2013) learnt the relationship between a number of gut microbiota and humans is more mutualistic instead of merely commensal.
- Clarke et al. (2014) found some human gut microorganisms help ferment dietary fibre into short-chain fatty acids, such as acetic acid and butyric acid, which are subsequently absorbed by the host. Furthermore, intestinal bacteria was discovered to be involved in synthesis of vitamin B and vitamin K, and metabolism of bile acids, sterols and xenobiotics.
- Quigley (2013) stated the composition of human gut microbiota alters over time, as well as during diet changes and overall health changes. Moreover, dysregulation of the gut flora is associated with a multitude of inflammatory and autoimmune diseases.
Classifications of the microbiota
- The microbial composition of the gut microbiota varies across the digestive tract. Guarner & Malagelada (2003) found relatively few bacterial species in the stomach and small intestine.
- In contrast, the colon has the greatest microbial density of any human-associated microbial community studied. Walker & Hoyles (2023) estimated between 1*1010 to 1*1011 bacterial cells per gram of intestinal matter.
- Sears (2005) approximated between 300 and 1000 different species of bacteria are represented in the colon, but Beaugerie & Petit (2004) claimed between 30 and 40 species of bacteria represent 99% of the species in the gut.
- Stephen & Cummings (1980) hypothesised 60% of the dry mass of faeces are composed of bacteria due to their abundance in the intestine. Lozupone et al. (2012) discovered the gut flora contains archaea, fungi, protists and viruses, but it's not fully understood what their main functions are in the gut.
- Sherwood, Willey & Woolverton (2013) found over 99% of the gut bacteria are anaerobes, whereas a majority of bacteria in the caecum are aerobic. Qin et al. (2010) estimated the gut flora contains approximately 100 times more genes than those in the human genome.
- Studies learnt a majority of the bacterial species in the gut are unable to be studied outside of their hosts because they are unable to be cultured. Tap et al. (2020) stated populations of microbial species can vary in spite of the small number of core microbial species shared by a majority of humans. O'Hara & Shanahan (2006) stated a person's microbiota remains generally constant over time, which may experience adaptations due to changes in age, diet and lifestyle.
- Khanna & Tosh (2014) described the 4 main bacterial phyla in the human gut are Baciliota (Firmicutes), Bacteroidota, Actinomycetota, and Pseudomonadota. Guarner & Malagelada (2003) found a majority of bacteria are categorised into the genera Bacteroides, Bifidobacterium, Clostridium, Eubacterium, Faecalibacterium, Peptococcus, Peptostreptococcus and Ruminococcus, and a minority of bacteria are categorised into the genera Escherichia and Lactobacillus.
- Cynthia Sears (2005) found 30% of all gut bacteria composes of species from the genus Bacteriodes, which indicates this genus plays essential roles in the host's functioning.
- Cui et al. (2013) discovered a number of fungal genera in the gut such as Aspergillus, Bullera, Candida, Galactomyces, Penicilium, Rhodotorula, Saccharomyces, Sclerotinia, and Trametes. Furthermore, Rhodotorula is often associated with inflammatory bowel disease and Candida is often associated with hepatitis B cirrhosis and chronic hepatitis B.
- Arumugam et al. (2011) defined enterotypes as a classification of living organisms based on its bacteriological ecosystem in the human gut microbiome not influenced by age, body weight, gender, or national divisions.
- Wu et al. (2011) suggested that the long-term diet has an effect on enterotype but more research is required to understand its impact.
Describe the composition of the gut microbiota
i. Stomach
- A majority of microorganisms are unable to survive the acidic environment inside the stomach. The main bacteria of gastric microbiota are classified into 5 major phyla: Actinobacteria, Bacteriodetes, Firmicutes, Fusobacteriota, and Proteobacteria.
- Nardone & Compare (2015) found the most common genera are Haemophilus, Prevotella, Rothia, Streptococcus, and Veillonella. When H. pylori enters the gut and interacts with the pre-existing gastric microbiota, it may have a significant effect on disease progression.
ii. Intestines
- The most prevalent microorganisms in the small bacteria are the gram-positive cocci and rod-shaped bacteria. Sherwood, Willey & Woolverton (2013) found the environment in the distal section of the small intestine is relatively alkaline, which tends to support gram-negative bacteria of the Enterobacteriaceae.
- The bacterial flora in the small intestine transmit regulatory signals that facilitate the development and utility of the gut. Without the regulatory signals, this leads to bacterial overgrowth, which can result in intestinal failure.
- Adams & Moss (2007) found roughly 99% of the large intestine and faecal flora are composed of obligate anaerobes such as Bacteroides and Bifidobacterium. The microorganism population of the large intestine can be affected by antibiotics, stress, and parasites.
- Studies by the University of Glasgow (2007) and Guarner & Malagelada (2003) concluded a majority of the intestinal flora is bacteria and comprises of 60% of faecal nitrogen. This indicates that the faeces are an ideal source of gut flora for tests and experiments about the colon by obtaining the nucleic acid from faecal specimens, and generating bacterial 16S nRNA gene sequences from bacterial primers.
- Braune & Blaut (2016) described the 5 most prominent phyla in the intestinal microbiota, which include Actinomycetota, Bacteroidota, Bacillota (Firmicutes), Pseudomonadota, and Verrucomicrobiota, with Bacteroidota and Bacillota comprising about 90% of the intestinal bacteria.
- Studies estimated between 300 and 1000 different species reside in the gut, however Beaugerie & Petit (2013) argued it was probable that about 99% of the bacteria consists of between 30 and 40 unique species, with Faecalibacterium prausnitzii (phylum firmicutes) being the most common species in healthy adult humans.
- Cynthia Sears (2005) hypothesised the relationship between gut flora and human is a mutualistic and symbiotic rather than simply commensal. The main functions of the microorganisms in the gut include:
- Fermentation of leftover energy substrates
- Using end products of metabolism (e.g. propionate and acetate) to train the immune system
- Inhibit growth of harmful species
- Regulation of gut development
- Production of vitamins such as biotin and vitamin K
- Production of hormones to facilitate lipid storage
- Ruth Ley (2010) suggested a correlation between obesity and the modifications and imbalances of the gut microbiota and its microbiome or gene collection. A 2023 study by the University of Glasgow hypothesised some species trigger infection or increase cancer risk for the host, which result in disease.
What factors can lead to variable gut microbiota?
i. Age
- Yatsunenko et al. (2012) found the adults had a considerably greater diversity of microbiota composition of faecal matter than in children, in spite of having lesser interpersonal differences than in children. Furthermore, a majority of microbiota's maturation into the composition observed in adult years occurs during the first 3 years of life.
- Changes in the microbiome composition consequently impacts the composition of the bacterial proteins created in the gut. Adult microbiomes tend to contain more enzymes involved in fermentation, methanogenesis and the metabolism of arginine, aspartate, glutamate, and lysine. In contrast, infant microbiomes contain more enzymes involved in cysteine metabolism and fermentation pathways.
ii. Geography
- Yatsunenko et al. (2012) studied the tradeoff of representation of the urease gene (Prevotella and the representation of genes encoding glutamate synthase / degradation or other enzymes responsible for degradation of amino acids or vitamin biosynthesis in different geographic populations. They discovered notable differences of this genetic trade-off between populations from Malawai, USA, or Amerindian origin.
- Furthermore, the USA population had a higher prevalence of enzymes encoding the degradation of glutamine, as well as vitamin and lipoic acid biosynthesis. In contrast, Malawai and Amerindian populations had a greater prevalence of enzymes encoding glutamate synthase, as well as excess representation of α-amylase in their gut microbiomes. Since the USA diets have higher fat content and less corn content than Amerindian or Malawian diets, it is thought to be a major determinant of the composition of gut bacteria.
- De Filippo et al. (2010) discovered the diets of children living in the rural village of Boulpon in Burkina Faso contained high levels of polysaccharides and plant proteins and low levels of fats and animal proteins compared to the diets of children living in Florence. The faecal bacteria of European children had higher prevalence of Firmicutes and decreased biodiversity, whereas the faecal bacteria of Boulpon children had higher prevalence of Bacteroidetes and had greater biodiversity.
- This indicates the gut microbiomes in African populations are capable of digesting typically indigestible plant polysaccharides, as well as decrease the incidence of non-infectious colonic diseases.
- It is demonstrated that sharing many common environmental exposures in a family is a compelling determinant of individual microbiome composition on a personal scale. Nonetheless, this effect elicits no genetic impact despite being consistently observed in culturally diverse populations.
iii. Malnourishment
- Daisy Jonkers found malnourished children have gut microbiota that are less mature and less diverse, which result in microbiome changes related to nutrient deficiency. Rytter et al. (2014) found malnourished children contain more pathogenic gut flora, and tend to have higher levels of yeast in their mouths and throats. Alcock et al. (2014) stated that changes in diet may result in changes in gut microbiota composition and diversity.
iv. Race and ethnicity
- Researchers for the American Gut Project and Human Microbiome Project discovered 12 microbe families varied in concentration according to the race or ethnicity of the individual. Renson & Herd (2020) pointed out the sample size of the American Gut Project is 1375 individuals and 90% of whom were white, which limited the cogency of these correlations.
- The Healthy Life in an Urban Setting (HELIUS) study conducted in Amsterdam discovered that individuals of Dutch ancestry contained the highest level of gut microbiota diversity, while individuals of South Asian and Surinamese descent had the lowest gut microbiota diversity. These findings implied that individuals of the same race or ethnicity shared similar microbiomes than individuals of different racial backgrounds.
v. Socioeconomic status
- A study in Chicago discovered individuals living in higher socioeconomic status (SES) neighbourhoods had higher gut microbiota diversity. In addition, those people had more prevalent Bacteroides bacteria in their gut flora.
- A study of UK twins also made similar findings with higher SES associating with higher gut diversity. More research is required to understand why this is the case.
How is gut microbiota acquired in infants?
- A child's microbiota is acquired through parent-to-child transmission and transfer from food, water, and other environmental sources to become similar to an adult's gut flora within the first 2 years of age.
- Matamoros et al. (2013) discovered microbial colonisation may occur in the human foetus with the presence of Lactobacillus and Bifidobacterium species in placental biopsies.
- A rodent study by Jiménez et al. (2005) showed the existence of bacteria in the amniotic fluid and placenta, as well as in the meconium of babies born by sterile C-section. Furthermore, Jiménez et al. (2009) orally administered a culture of bacteria to pregnant mice, and discovered the same bacteria present in the offspring, which is likely due to transmission between the digestive tract and amniotic fluid via the blood stream.
- Perez-Muñoz et al. (2017) advised that it is not well understood where these intrauterine bacteria originated, whether they are alive, and their actual functions. These findings defy the popular belief that the gut of a normal foetus is sterile.
- Sommer & Bäckhed (2013) stated that bacteria from the mother and the surrounding environment colonise the infant's gut during and after birth. Although it is not known where that bacteria originated from, Adlerberth & Wold (2009) hypothesised the bacteria may originate from the birth canal, transmitted from other people (e.g. parents, siblings, hospital workers), and the general environment with the infant interacts.
- A 2019 ScienceDaily report demonstrated that the microbiome of babies born vaginally is remarkably different compared to the microbiomes of babies delivered by C-section. Furthermore, babies born vaginally receive a majority of their gut bacteria from their mother, whereas babies born by C-section receive their gut bacteria from hospital environments.
- During the first year of life, the composition of the gut microbiome is fairly simple, which increases in diversity over time, leading to a variety of microbiome compositions across individuals.
- The initial bacterial population consists of mainly facultative anaerobic organisms, which is thought to reduce the oxygen levels in the gut. This would, in turn, allow anaerobic bacteria such as Actinomycetota, Bacillota, and Bacteroidota to become manifested and thrive.
- Babies that are breastfed have mainly bifidobacteria in their gut microbiome, probably due to the presence bifidobacterial growth factors and prebiotic substances in breast milk.
- Mady et al. (2023) found breast milk contains high levels of Immunoglobulin A (IgA) to support the tolerance and regulation of the baby's immune system. In contrast, Fanaro et al. (2007) discovered the infants fed with baby formula contained a diverse microbiota, with high levels of Bacteroides, bifidobacteria, clostridia, enterobacteriaceae and enterococci.
- Mueller et al. (2015) found evidence that antibiotics, C-sections and baby formula influences the gut microbiome composition in babies. Furthermore, C-sections were demonstrated to disrupt the transmission of bacteria from mother to offspring, which increases the offspring's risks of experiencing diseases such as asthma, celiac disease, type 1 diabetes.
- Yassour et al. (2016) discovered children that were administrated antibiotics had floral communities that decreased in diversity and in stability. A number of methods thought to restore the gut microbiome have been studied, with the most common method being exposing the infant to maternal vaginal substances, and oral probiotics.
Describe the functions of the gut microbiota
- Gibson & Roberfroid (1995) hypothesised the gut flora has 3 key functions: (1) direct defence against pathogens, (2) strengthen the host's defence by developing the maintaining the intestinal epithelium where antibody production occurs, and (3) metabolising usually indigestible substances in food.
- Wang & Kasper (2014) discovered the gut flora is also involved in training the developing immune system, as well as the gut-brain axis.
i. Direct inhibition of pathogens
Yoon et al. (2014) stated the gut microbiome is involved in defence against pathogens by extensively colonising the space, utilising all available nutrients, and by producing compounds called cytokines that eliminate or inhibit foreign organisms that intend to compete for nutrients with it. Note that different strains of gut bacteria produce different cytokines. If the gut flora is disrupted, competing organisms such as Clostridium difficile would become established rather than be in a state of latency.
ii. Development of the enteric production and immune system
- Sommer & Bäckhed (2013) found that gut flora allows the developing intestinal epithelium and the intestinal mucosal barrier to be tolerant or supportive of commensalistic microorganisms to an extent, as well as produce a barrier to pathogenic microorganisms.
- Faderl et al. (2015) stated that goblet cells proliferate to thicken the mucosa layer, which adds an outer mucosa layer in which commensal microorganisms can established and feed, as well as an inner layer that are impermeable to these microorganisms.
- The development of gut-associated lymphoid tissue (GALT) coincides with the development and establishment of the gut microbiome. GALT forms part of the intestinal epithelium that perceives and responds to pathogens, appears and matures during the time of the establishment and development of the gut flora.
- This GALT is tolerant to mainly gut microbiome species, but not to other microorganisms outside of the gut. In addition, GALT typically becomes tolerant to food the infant interacts with, as well as the digestive products of food, and the metabolites of the gut flora produced from food.
- A number of bacterial species in the gut flora demonstrated to stimulate the immune system to produce cytokines selectively. e.g. Bacteroides fragilis and some Clostridia species trigger an anti-inflammatory response, while a number of segmented filamentous bacteria facilitate the production of inflammatory cytokines.
- The gut flora play major roles in the regulation of the production of antibodies by the immune system. Peterson & Artis (2014) found the gut flora triggers NF-κB signalling by intestinal cells, leading to further signalling molecules being produced, resulting in B cells switching class to IgA.
- Honda & Littman (2016) showed that IgA aids in diversifying the gut microbiome and supports the elimination of bacteria that trigger inflammatory responses. IgA fundamentally maintains a healthy environment between the host and gut bacteria.
- The metabolites produced by the gut bacteria can influence the cells of the immune system. Levy, Thaiss & Elinav (2016) found that short-chain fatty acids (SCFA) produced by some gut bacteria via fermentation can trigger a sudden increase in the production of innate immune cells such as basophils, eosinophils, and neutrophils. These immune cells serve to limit the infection spreading throughout the host's body.
iii. Metabolism
This diagram illustrates the biosynthesis of bioactive compounds (e.g. indole and other derivatives) from tryptophan by gut bacteria.
- (Right) A study by Zhang & Davies (2016) found bacteria that express tryptophanase play a role in producing indole from tryptophan.
- (Left) Wikoff et al. (2009) found clostridium sporogenes metabolises tryptophan into indole and subsequently a highly potent neuroprotective antioxidant called 3-indolepropionic acid (IPA), which scavenges hydroxyl radicals.
- IPA binds to the pregnane X receptor (PXR) in intestinal cells, which then facilitates mucosal homeostasis and barrier function.
- After being absorbed from the intestine and circulated to the brain, IPA elicits a neuroprotective effect against cerebral ischaemia and Alzheimer's disease.
- (Middle) Lactobacillaceae (Lactobacillus s.l.) species metabolise tryptophan into indole-3-aldehyde (I3A), which binds to aryl hydrocarbon receptor (AhR) in intestinal immune cells. This increases the production of interleukin-22 (IL-22).
- In addition, the liver can metabolise indole into indoxyl sulfate, which is toxic in high levels and linked to vascular disease and renal dysfunction.
- Known as activated charcoal, AST-120 is an intestinal sorbent that adsorbs indole, which results in reduction of indoxyl sulfate in blood plasma.
- Gut flora play an important role in supporting the human body using the undigested carbohydrates it consumes. Clarke et al. (2014) found some species of gut bacteria contain enzymes that human cells lack for disintegrating a number of polysaccharides. In addition, gut bacteria aid humans in digesting fibre, starches, oligosaccharides, and sugars such as lactose in cases of lactose intolerance and sugar alcohols, as well as mucus, and proteins.
- Furthermore, rodents living in a sterile environment and lacking a gut microbiota had to consume 30% more calories in order to be at the same weight as their typical counterparts.
- Glenn Gibson (2004) found bacteria converts carbohydrates into short-chain fatty acids by a fermentation process called saccharolytic fermentation. Examples of those short-chain fatty acids include acetic acid, butyric acid, and acetic acid. These substances are subsequently utilised by host cells, which becomes a major source of energy and nutrients. For example, acetic acid is utilised by muscle, butyric acid offers energy to gut cells, and propionic acid facilitates ATP production in liver.
- Beaugerie & Petit (2004) found other by-products of fermentation include gases and organic acids, such as lactic acid.
- O'Hara & Shanahan (2006) found the gut microbiome produces vitamins such as biotin and folate, and facilitates absorption of dietary minerals, such as calcium, iron, and magnesium.
- Rajilić-Stojanović & De Vos (2014) discovered Methanobrevibacter smithii is the most abundant archaeal species that synthesises methane in the gut microbiota.
- Hill (1997) found the gut flora synthesises vitamin B12 and vitamin K, which aren't typically synthesised by the body.
a. Cellulose decomposition
- Bacteria known to degrade cellulose such as Ruminococcus) are common among the great apes, ancient human societies, hunter-gatherer communities, and modern rural communities, but rare in industrialised communities.
- Bacterial species discovred in primates can break down specific plant fibres such as maize, rice, and wheat, as well as chitin, a polymer in insects. Moraïs et al. (2024) suggested the transition toward western lifestyles may have resulted in the reduction of these bacterial species in the human gut.
b. Pharmacomicrobiomics
- El Rakaiby et al. (2014) estimated the total number of microbial cells in the human body is approximately over 100 trillion, which significant outnumbers the number of Homo sapiens cells (~ 10 trillion). Nevertheless the human metagenome can vary significantly between individuals.
- Cho & Blaser (2012) found potential for interactions between drugs and an individual's microbiome. Kumar et al. (2019) stated those interactions include drugs changing the composition of the human microbiome, drug metabolism by microbial enzymes regulating the drug's pharmacokinetic profile, and microbial drug metabolism influencing a drug's clinical efficacy and toxicity profile.
- Sousa et al. (2008) found gut microbiota can metabolise other xenobiotics including drugs, food toxicants, and phytochemicals. Haiser et al. (2013) thought the microbial metabolism of drugs may inactivate them.
c. Role in drug metabolism
- Koppel et al. (2017) described the gut microbiota as an enriched population of various genes with vast biochemical potential to moderate drugs.
- Spanogiannopoulos et al. (2013) found drug metabolism can be influenced by the gut microbiota both directly and indirectly. Maini Rekdal et al. (2019) described the direct mechanism is moderated by the microbical enzymes that can change the chemical structure of the administered drugs.
- On the other hand, Dempsey & Cui (2019) described the indirect mechanism is regulated by the microbial metabolites that influence the expression of host metabolising enzymes e.g. cytochrome P450.
- Researchers concluded the effects of the gut microbiota on the pharmacokinetics and bioavailability of the drug are varied. They found the gut microbiota could activate inactive drugs such as lovastatin, or inactivate active drugs such as digoxin, or result in drug toxicity such as irinotecan.
- Maini Rekdal et al. (2019) discovered the gut microbiota can produce and secrete enzymes that are capable of metabolising drugs such as microbial biotransformation of L-dopa by decarboxylase and dehydroxylase enzymes.
- Conversely, Dempsey & Cui (2019) discovered the gut microbiota can change the metabolism of the drugs by regulating the host drug metabolism. This mechanism is thought to be moderated by microbial metabolites or by regulating host metabolites that alter the expression of host metabolising enzymes.
- Yoo et al. (2014) found lovastatin (a lactone pro-drug that reduces cholesterol) is partially activated by the human gut microbiota, which produce active acid hydroxylated metabolites.
- On the contrary, Koppel et al. (2018) found digoxin is inactivated by eggerthella lanta, a bacterial specimen of the gut microbiome. This bacteria contains a cytochrome-encoding operon that is up-regulated by digoxin and is correlated with inactivation of digoxin.
- Alexander et al. (2017) discovered the gut microbiota can regulate the efficacy and toxicity of chemotherapeutic agents such as irinotecan. Brandi et al. (2006) explained this effect originated from the microbiome-encoded β-glucuronidase enzymes, which regain the active form of the irinotecan resulting in gastrointestinal toxicity.
d. Secondary metabolites
- Multiple researchers supported the theory that the gut microbiome is capable of producing unique secondary metabolites that may be developed from the metabolic conversion of dietary foods such as fibres, endogenous biological compounds such as indole or bile acids.
- Yang et al. (2020) found microbial metabolites particularly short chain fatty acids (SCFAs) and secondary bile acids (BAs) are involved in human health and disease.
- The secondary metabolites are produced by gut bacteria biotransforming primary bile acids (e.g. cholic acid (CA) and chenodeoxycholic acid (CDCA)) into secondary bile acids (e.g. ithocholic acid (LCA) and deoxy cholic acid (DCA) respectively).
- Jones et al. (2008) described the process of primary bile acids being produced by hepatocytes and then stored in the gallbladder. Next, the gut microbiota subsequently break down these metabolites into secondary metabolites, which increases its hydrophobicity. Moreover, bile salt hydrolases (BSH) are maintained across gut microbiota phyla such as Actinobacteria, Bacteroides, and Firmicutes, which plays a role in establishing the first step of secondary bile acids metabolism.
- Thanissery et al. (2017) demonstrated that the secondary bile acids such as DCA and LCA inhibits Clostridium difficile from germinating and growing.
e. Dysbiosis
- Dysbiosis is defined as an imbalance of microbial species due to decreases in microbial diversity within certain bodily microbiomes. Cao et al. (2017) suggested a correlation between dysbiosis and the development of intestinal cancer. Moreover, there is a correlation between the secondary bile acid deoxycholic acid and changes to the gut microbiome, which result in increase in intestinal carcinogenesis.
- Bernstein & Bernstein (2023) found the colon's increased exposure to secondary bile acids manifesting from dysbiosis led to DNA damage, which resulted in carcinogenic mutations in the cells of the colon. Moreover, it is suggested that the higher density of bacteria in the colon (~ 1012 / ml) subject to dysbiosis increases the incidence of cancer in the colon by a factor of 10 compared to the lower density of bacteria in the small intestine (~ 102 / ml).
iv. Gut-brain axis
- The gut-brain axis is referred as the bidirectional biochemical signalling that occurs between the gastrointestinal tract (GIT) and the central nervous system (CNS). Mayer et al. (2014) stated the "microbiota-gut-brain axis" involves the gut microbiota in the biochemical signalling processes that occur between the GIT and the CNS.
- Broadly speaking, the gut-brain involves numerous systems such as the CNS, the hypothalamic-pituitary axis (HPA), neuroendocrine system, neuroimmune system, sympathetic and parasympathetic branches of the autonomic nervous system (ANS), the enteric nervous system (ENS), and the gut microbiota.
- In 1904, Pavlov was the first researcher to demonstrate brain-gut interactions. He corroborated the cephalic phase of digestion that sensory signals, such as the smell and sight of food, triggered the release of gastric and pancreatic secretions.
- A 2015 review by Marilia Carabotti reported that the microbiome affects the CNS by modulating the brain chemistry and regulating the neuro-endocrine systems associated with anxiety, stress response, and memory.
- The bidirectional communication between the GIT and the CNS is conducted by endocrine, humoral, immune and neural signalling. Cryan et al. (2019) hypothesised that the gut microbiota affects the brain function by producing a number of chemicals such as chemokines, cytokines, endocrine messages, neurotransmitters, neuropeptides, and microbial metabolites such as branched chain amino acids, short-chain fatty acids, and peptidoglycans.
- Kaelberer et al. (2020) found the intestinal microbiome can subsequently transfer these products to the brain via the bloodstream, endocrine cells, nerves, neuropod cells, etc. This, in turn, supposedly influences several metabolic processes.
- Cowan et a. (2017) supported the hypothesis of signalling between the amygdala, the hippocampus, and the prefrontal cortex, which serve as a key node in the gut-brain behavioural axis.
- Chen et al. (2021) found disruptions to the gut-brain axis correlates with a number of disorders such as irritable bowel syndrome, anxiety, autism, depression and schizophrenia.
i. Enteric Nervous System (ENS)
- The enteric nervous system consists of a mesh-like system of neurons that controls the function of the gastrointestinal system. Since the ENS can function autonomously, scientists referred to it as "the second brain".
- The ENS typically communicates with the CNS via the parasympathetic (e.g. vagus nerve) and sympathetic (e.g. prevertebral ganglia). Li & Owyang (2003) demonstrated that the ENS continues to function normally after severing the vagus nerve. In vertebrates, the ENS includes afferent neurons, efferent neurons, and interneurons, all of which carry reflexes when there is no CNS input.
- The sensory neurons perceive mechanical and chemical stimuli, whereas the motor neurons play a role in peristalsis and churning of food inside the intestines.
- The ENS produces more than 30 neurotransmitters, most of which are also produced by the CNS, e.g. acetylcholine, dopamine, and serotonin. Martinucci et al. (2015) estimated more than 90% of the body's serotonin and about 50% of the body's dopamine are situated in the gut.
ii. Gut-brain Integration
- The gut-brain axis plays an essential role in maintaining homeostasis and is monitored through the CNS and ENS, as well as the endocrine, immune, neural, and metabolic pathways, and the hypothalamic–pituitary–adrenal axis (HPA axis). Since there is an association between the gut microbiota and brain function, the term expands to become the "microbiome-gut-brain axis".
- Sudo & Chida (2004) conducted a germ-free mice study and found an amplified HPA axis response to stress, compared to non-germ-free mice.
- Petra et al. (2015) found the gut microbiota can secrete a diverse range of neurotransmitters, such as acetylcholine, catecholamines, γ-aminobutyric acid, histamine, melatonin, and serotonin, which play important roles in moderating peristalsis and sensation in the gut.
- When diet, drugs, or disease affects changes in the composition of the gut, it may result in changes in the levels of cytokines, some of which may impact brain function. The gut microbiota also produce molecules that directly activates the vagus nerve, which transfers information about the condition of the intestines to the brain.
- Factors such as hypothalamic-pituitary-adrenal axis, cholinergic anti-inflammatory pathway, feelings of hunger and satiety, and the presence of absence of food in the gut can impact the gut composition, the gut epithelium and the gut microbiota.
- Melbye et al. (2019) suggested the process of autoimmunity is moderated by the gut microbiota, which ferment dietary fibre and starch. In addition, the fermentation process produces short chain fatty acids (SCFAs) such as acetate, butyrate, and propionate.
iii. Gut-brain-skin axis
- In 1930, Pillsbury Stokes published a theory that associate gastrointestinal pathways with anxiety, depression, and skin conditions such as acne. That paper suggested that emotional states may change the intestinal microbiota, which may increase intestinal permeability and thus contribute to systemic inflammation.
- Bowe & Logan (2011) found the gut microbiota and oral probiotics are associated with mood, tissue lipid content, glycemic control, oxidative stress, and systemic inflammation.
What can change the balance of the microbiota?
i. Antibiotics
- Carman et al. (2014) stated that broad-spectrum antibiotics can change the concentration of gut bacteria, which impact the host's health and ability to digest food. Furthermore, antibiotics were discovered to increase the levels of antibiotic-resistant bacteria, which can trigger illnesses that are difficult to treat with conventional antibiotics.
- Beaugerie & Petit (2004) found that antibiotics can directly irritate the bowel to cause diarrhoea, which alters the gut bacteria levels, or facilitates growth of pathogenic bacteria.
- When the levels and species of gut microbiota change, it decreases the body's ability to ferment carbohydrates and metabolise bile acids, which may result in diarrhoea. If carbohydrates aren't disintegrated, they absorb excess water and result in runny stools, or lack of SCFAs produced by the gut microbiota can result in diarrhoea.
- Guarner & Malagelada (2016) found decreases in levels of native bacterial species can disturb their ability to inhibit the growth of harmful species such as C. difficile and Salmonella Kedougou. Ultimately, this would result in overgrowth of these bacterial species, leading to diarrhoea.
- Hvas et al. (2020) found the faecal microbiota transplantations to treat C. difficile infections yielded success rates of about 90%, which minimal side-effects. Brandt et al. (2011) conjectured the efficacy of this treatment is due to restoration of bacterial balances of bacteroides and firmicutes classes of bacteria.
- In several illnesses, antibiotic usage, as well as ischemia of the gut, lack of eating, and immune harm can change the composition of the microbiome. Knight & Girling (2003) suggested selective digestive tract decontamination as a possible treatment to kill only pathogenic bacteria and facilitate the re-manifestation of healthy bacteria.
- Cho et al. (2012) found that not only antibiotics change the bacterial population of the gut microbiota, but also change the metabolic interactions within the bacteria community, alter caloric intake with the use of carbohydrates, and influence horminal, immune and metabolic homeostasis.
- Schneiderhan et al. (2016) discovered evidence of probiotics containing Lactobacillus species may reduce the incidence of antibiotic-associated diarrhoea and probiotics with Saccharomyces may hinder Clostridium difficile infection.
ii. Pregnancy
The gut microbiota of a pregnant woman changes during the course of the pregnancy, which are similar to those observed in metabolic syndromes such as diabetes. Mueller et al. (2015) found the gut microbiota is similar to the gut microbiota in the mother during the first-trimester. Moreover, as the pregancy advances from the 1st to the 3rd trimeter, the diversity of the gut microbiome reduces, and the levels of a number of certain bacteria species increases.
iii. Probiotics, prebiotics, synbiotics, and pharmabiotics
- Hill et al. (2014) described probiotics as microorganisms that are thought to provide health benefits to the host after consumption.
- Hutkins et al. (2016) described prebiotics as non-digestible, fibre compounds that pass undigested through the upper section of the GIT and trigger the growth or activity of beneficial gut flora by serving as a substrate for them.
- Pandey et al. (2015) defined synbiotics as food ingredients or dietary supplements that contain both probiotics and prebiotics in a synergist manner.
- Broeckx et al. (2016) defined 'pharmabiotics' as pharmaceutical productions of probiotics, prebiotics, or synbiotics.
- For example, Sleator & Hill (2009) described probiotics can be genetically engineered or optimised in order to achieve the optimal performance in terms of shelf life, survival in the digestive tract etc.
Studies identified a number of bacterial species that reduced symptoms:
-- Bifidobacterium breve
-- Bifidobacterium infantis
-- Enterococcus faecium
-- Lactobacillus plantarum
-- Lactobacillus reuteri
-- Lactobacillus rhamnosus
-- Lactobacillus salivarius
-- Propionibacterium freudenreichii
-- Saccharomyces boulardii
-- Escherichia coli Nissle 1917
-- Streptococcus thermophilus
iv. Fecal floatation
Levitt & Duane (1972) estimated that 10-15% of faeces consistently float in toilet water due to gas (i.e. floaters), while the remainder sink in toilet water due to lack of gas (i.e. sinkers). Although conventional mice typically produce 'floaters', gnotobiotic germfree mice with no gut microbiota produce 'sinkers'. Furthermore, gut microbiota colonisation in germfree mice resulted in food transformation to microbial biomass and enrichment of several gas-producing bacterial species that turn 'sinkers' into 'floaters.
v. Exercise
Clauss et al. (2021) suggested a strong correlation between gut microbiota and exercise after discovering different health effects resulted from varying levels of exercise (moderate vs. intense). Nevertheless, this correlation depends on intensity of the exercise and training status.
Describe the blood supply of the digestive system
- The digestive system mainly receives blood from the coeliac artery, which is the 1st major branch from the abdomen aorta. The 3 main divisions of the coeliac artery are the left gastric artery, the common hepatic artery and the splenic artery.
- The coeliac artery primarily supplies oxygenated blood to the liver, stomach, spleen and the upper third of the duodenum (to the sphincter of Oddi) and the pancreas.
- A majority of the deoxygenated blood travels to the liver via the portal venous system, where it gets processed and detoxified before it returns to systemic circulation via the hepatic veins.
- The subsequent branch from the abdominal aorta is the superior mesenteric artery, which supplies oxygenated blood to areas of the digestive tract derived from the midgut. This includes the distal two thirds of the duodenum, jejunum, ileum, cecum, appendix, ascending colon, and the proximal two thirds of the transverse colon.
- The last branch is the inferior mesenteric artery, which provides oxygenated blood to the areas of the digestive tract derived from the hindgut. This includes the distal third of the transverse colon, descending colon, sigmoid colon, rectum, and the anus (situated above the pectinate line).
- Waaler & Toska (1999) stated blood flow to the digestive tract reaches its maximum 20–40 minutes after a meal and lasts for 1.5–2 hours.
Describe the nerve supply of the digestive system
- The ENS contains approximately 100 million neurons embedded in the peritoneum, the lining of the GIT stretching from the oesophagus to the anus.
- Bowen (2008) described these neurons are assembled into 2 plexuses - the myenteric (or Auerbach's) plexus and the submucosal (or Meissner's) plexus. The myenteric plexus is situated between the longitudinal and the smoother muscle layers, and the submucosal plexus is situated between the circular smooth muscle layer and the mucosa.
- The vagus nerve provides parasympathetic innervation to the ascending colon, whereas the splanchnic nerves provides sympathetic innervation.
- A majority of the digestive tract is innervated by 2 large coeliac ganglia, with the upper section of each ganglion connected by the greater splanchnic nerve and the lower sections connected by the lesser splanchnic nerve.
You can appreciate the complexity of the processes of digestion in the human body because the living cells require a consistent supply of diverse, energy-rich organic molecules from the environment in order to function properly. It is incredible how our bodily organs can break down any kind of food we consume into the simplest biochemical forms such as glucose (sugars), amino acids (proteins), or fatty acids (fats). Then these simpler biochemicals are absorbed from the small intestine and into the bloodstream.
This raises a number of questions regarding the next steps following digestion.
- But how does the body convert that digested food into useful energy and new cells to grow the body and repair damage cells?
- How does the body direct this energy to the appropriate cellular locations that need replenishment, growth, or repair?
- How do the living cells take up that energy and use it for normal function?
- How does it impact metabolic rate and homeostasis?
I will delve into the processes of metabolism, catabolism, anabolism in another post.